Tau.Neutrino said:
How many neutrons can you cram into an atom? More than physicists thought
Physicists in Japan have blasted out the heaviest calcium nuclei ever seen—each containing the 20 protons needed to make the element, but with a huge number—40—of neutrons. That’s twice as many neutrons as in calcium’s most common form, and a couple more than the previous record. The finding suggests it may be possible to cram even more neutrons into nuclei than previously thought, and it could have implications for the theory of neutron stars.
more…
Calcium-48 with 20 protons and 28 neutrons is the standard high-neutron isotope ion used in making superheavy elements. It’s doubly magic, and has such a long half life that it’s effectively stable.
Calcium-57 has been found before, with 20 protons and 37 neutrons. It has a half life near 5 milliseconds.
> Japan’s RIKEN laboratory in Wako and Michigan State University in East Lansing have produced a batch of new neutron-rich nuclei that suggest the drip line is farther off than many theories predict, they reported last week in Physical Review Letters. The team hunted in the neighborhood of calcium because its magic number of protons already imbues it with stronger binding.
Heck, that’s serious news. It could upset models of isotope synthesis in supernovae, which is where our solar system got its elements.
> The discovery of the important neutron-rich nucleus calcium-60 and seven others near the limits of nuclear stability is reported from the fragmentation of a
345 MeV zirconium-70 projectile beam on beryllium-9 targets at the radioactive ion-beam factory of the RIKEN Nishina Center. The produced fragments were analyzed and unambiguously identified using the BigRIPS two-stage in-flight separator. The eight new neutron-rich nuclei discovered, phosphorus-47, sulfur-49, chlorine-52, argon-54, potassium-57, calcium-59, calcium-60 and scandium-62 are the most neutron-rich isotopes of the respective elements. In addition, one event consistent with potassium-59 was registered. The results are compared with the drip lines predicted by a variety of mass models and it is found that the models in best agreement with the observed limits of existence in the explored region tend to predict the even-mass Ca isotopes to be bound out to at least calcium-70.
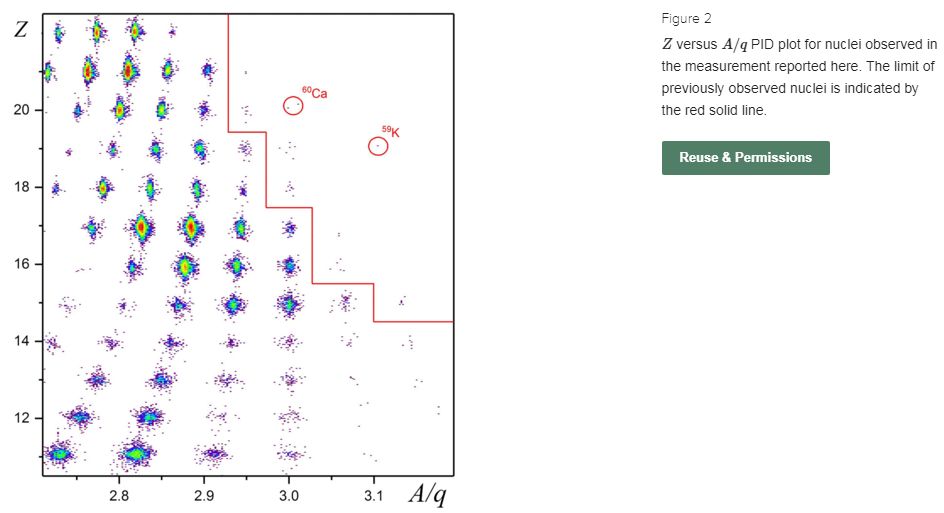
Apparently, from their Figure 2, calcium-58 is already known. But even it hasn’t appeared in wikipedia yet.