Do stars create life?
> So, which came first – the chlorophyll, the NADP+, the protein ferredoxin-NADP+ reductase, or the phospholipid membrane?
Organic bilayer membranes are older than life itself, much older.
“NADPH is an essential electron donor in all organisms, including archaea and bacteria”. See https://www.ncbi.nlm.nih.gov/pubmed/26284036
“The dehydrogenase reactions of the oxidative pentose phosphate pathway (oxPPP), the Entner–Doudoroff (ED) pathway, and the isocitrate dehydrogenase step of the tricarboxylic acid (TCA) cycle have been considered the major sources of NADPH. However, the importance of other NADPH-generating enzymes, such as transhydrogenases, glucose dehydrogenases, and non-phosphorylating glyceraldehyde 3-phosphate dehydrogenase (GAPN), is becoming clear.”
“To assess the feasibility of various NADPH-generating reactions, we calculated the Gibbs energies (ΔrG′m) using the biochemical thermodynamics calculator eQuilibrator 2.0”
I wish I’d had that calculator back when I was looking into the calculation of the origin of macromolecule precursors of life.
So, I can answer the above question about photosynthesis.
Membranes came first. Generated abiologically.
NADPH came second. We know that it can be generated abiologically in a cyanide (HCN) atmosphere with phosphorus. (Note that a cyanide atmosphere requires a loss of environmental hydrogen and oxygen).
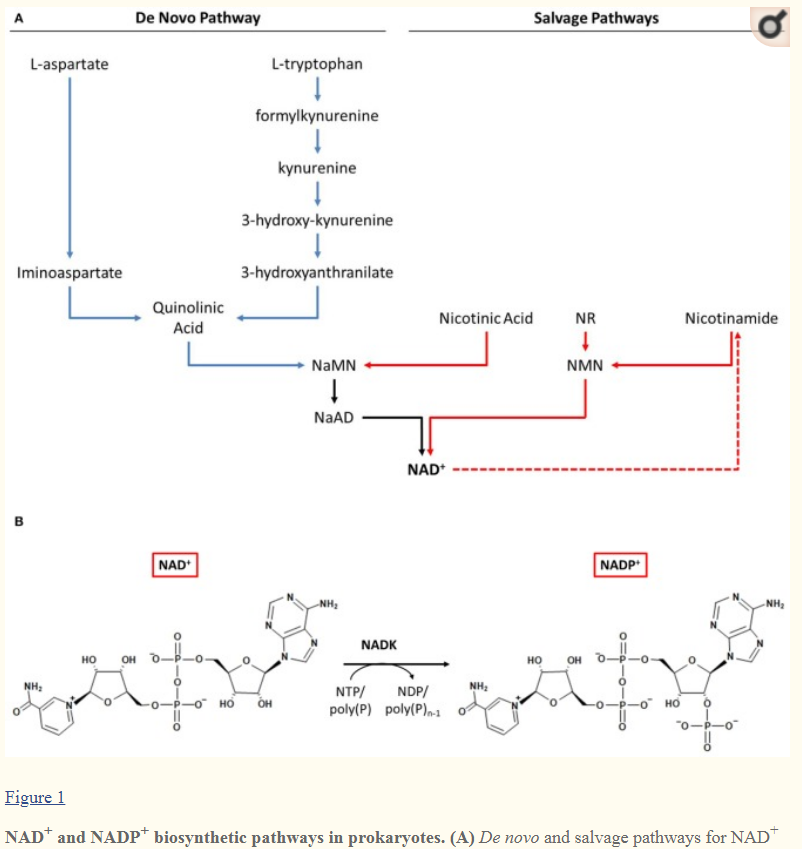
Proteins that make NADPH came third. There are 12 different proteins known, which evolved slowly to greater effectiveness over hundreds of millions of years.
Chlorophyll came last, and fitted neatly into a system that was already in operation.
——————
The slow development of a complex chemical cycle like photosynthesis argues for a similar slow development of the complex chemical cycle that we call life. The early prebiotic stages of this evolution have all been lost, so have to be reconstructed by experiment and theory.