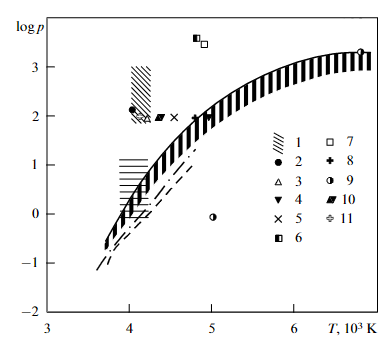
“E.I. Asinovsky, A.V. Kirillin, A.V. Kostanovskii, Experimental investigation of the thermal properties of carbon at high temperatures and moderate pressures. Phys. Uspekhi 45, 869–882 (2002)” via Google scholar and SciHub.
Figure 3 helps to make sense of this by looking at higher pressures. Results of literature search together with author’s measurements. The near vertical line is melting point, the line to the right is boiling point. The lowest points are at 1 bar. The scatter in temperature is nearly independent of pressure.
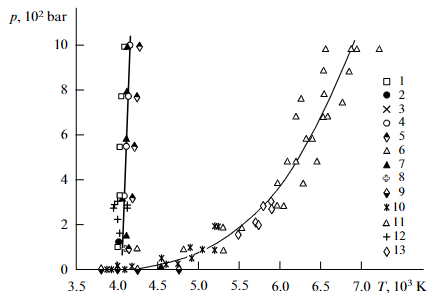
This allows me to say that the boiling point of carbon at 1 bar is 4,000 +- 250 Kelvin. The melting point of graphite (but see below) at 1 bar, extrapolated down from 80 bar, is 4,050 +- 100 Kelvin.
This helps to explain why some researchers say that carbon sublimes, and why others say that carbon melts and then boils.
But why is it so difficult to measure, when compared to metals?
Redrawn Fig. 3 in a form suitable for Wikipedia.
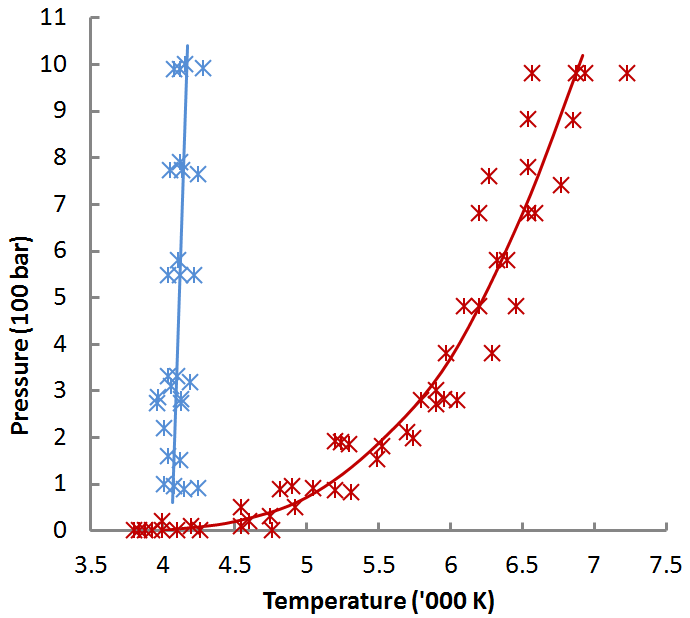
Does that match up with what i found before, ie.
4,827 °C boiling point, melting point 3,500 °C
3,642 °C, sublimes
3,825 °C boiling point, melting point 3,550 °C
3,825 °C, sublimes
4,832 °C boiling point, melting point 3,367 °C
3,630 °C, sublimes, triple point 4,330 ± 300 °C
4,027 °C boiling point, melting point 3,550 °C for diamond
4,830 °C boiling point, melting point 3,500 °C for graphite
Not really, the range from Fig. 3 is 3,800 to 4,760 K.
As against 3.900 to 5,105 K from the web. That top temperature is a lot hotter.
There’s also a disagreement for melting point, 4,050 +- 100 K from Fig. 3.
As against 3,640 to 3,915 K from the web, with an outlier of 4,100 K.
What a mess. All the researchers are missing something here. I wonder what it is?