1 ppm in this case is 1 molecule of O2 in a million molecules of dry air.
What’s that in gigatons of O2?
1 ppm in this case is 1 molecule of O2 in a million molecules of dry air.
What’s that in gigatons of O2?
mollwollfumble said:
1 ppm in this case is 1 molecule of O2 in a million molecules of dry air.What’s that in gigatons of O2?
O2 has a MW of about 16.
So the mass of O2 in this sample will be given by
m = m~sample~ * P~oxygen * MW~oxygen~ / MW~sample
where m~sample~ = the mass of the entire sample
P~oxygen = the molar fraction of O2 = 1/1000000 in this case
MW~oxygen = the molecular weight of O2 = about 16
MW~sample = the mean molecular weight in the sample
mollwollfumble said:
1 ppm in this case is 1 molecule of O2 in a million molecules of dry air.What’s that in gigatons of O2?
I am not sure what you are asking here.
The mass of one O2 molecule is about 32/6.022E23 grams = 5.31E-23 g = 5.31e-38 Gt.
Is that what you wanted?
>>1 ppm in this case is 1 molecule of O2 in a million molecules of dry air. What’s that in gigatons of O2?
> Is that what you wanted?
I didn’t express myself clearly.
1 ppm O2 is one O2 molecule in 1,000,000 molecules of air.
Mean mass of atmosphere is 5.148×1018 kg
Molecular weight of O2 is 32
Mean molecular weight of air molecule is 29.
So mass of 1 ppm O2 is 5.148×1018 /1,000,000*32/29 kg = 5.68×1012 kg
= 5.68 gigatonnes. Plus or minus a bit.
I’m using this to do a mass balance on the atmosphere, ignoring lithosphere weathering.
Oxygen removed by CO2 burning – Oxygen depletion in the atmosphere = Net oxygen released by growing forests.
Which gives us a close estimate of net global biosphere growth every year. I hope.
mollwollfumble said:
>>1 ppm in this case is 1 molecule of O2 in a million molecules of dry air. What’s that in gigatons of O2?> Is that what you wanted?
I didn’t express myself clearly.
1 ppm O2 is one O2 molecule in 1,000,000 molecules of air.
Mean mass of atmosphere is 5.148×1018 kg
Molecular weight of O2 is 32
Mean molecular weight of air molecule is 29.So mass of 1 ppm O2 is 5.148×1018 /1,000,000*32/29 kg = 5.68×1012 kg
= 5.68 gigatonnes. Plus or minus a bit.I’m using this to do a mass balance on the atmosphere, ignoring lithosphere weathering.
Oxygen removed by CO2 burning – Oxygen depletion in the atmosphere = Net oxygen released by growing forests.
Which gives us a close estimate of net global biosphere growth every year. I hope.
Wouldn’t it be easier to look at the increase in CO2, rather the decrease in O2?
The Rev Dodgson said:
mollwollfumble said:
>>1 ppm in this case is 1 molecule of O2 in a million molecules of dry air. What’s that in gigatons of O2?> Is that what you wanted?
I didn’t express myself clearly.
1 ppm O2 is one O2 molecule in 1,000,000 molecules of air.
Mean mass of atmosphere is 5.148×1018 kg
Molecular weight of O2 is 32
Mean molecular weight of air molecule is 29.So mass of 1 ppm O2 is 5.148×1018 /1,000,000*32/29 kg = 5.68×1012 kg
= 5.68 gigatonnes. Plus or minus a bit.I’m using this to do a mass balance on the atmosphere, ignoring lithosphere weathering.
Oxygen removed by CO2 burning – Oxygen depletion in the atmosphere = Net oxygen released by growing forests.
Which gives us a close estimate of net global biosphere growth every year. I hope.
Wouldn’t it be easier to look at the increase in CO2, rather the decrease in O2?
:-)
I was hoping you’d pick me up on that. CO2 leaves the atmosphere in two ways – to the biosphere and by solution in the oceans. The two ways are of almost equal magnitude.
For O2, the effect of O2 absorption or release by the oceans (abiologically) is negligible. That was an insight I picked up from the third IPCC report. I hadn’t realised it before then.
So for CO2, the equation is:
CO2 from burning – CO2 increase in the atmosphere = CO2 uptake by growing forests + CO2 uptake from oceans.
Can’t get forest growth on its own without the mass balance on O2.
mollwollfumble said:
The Rev Dodgson said:
mollwollfumble said:
>>1 ppm in this case is 1 molecule of O2 in a million molecules of dry air. What’s that in gigatons of O2?> Is that what you wanted?
I didn’t express myself clearly.
1 ppm O2 is one O2 molecule in 1,000,000 molecules of air.
Mean mass of atmosphere is 5.148×1018 kg
Molecular weight of O2 is 32
Mean molecular weight of air molecule is 29.So mass of 1 ppm O2 is 5.148×1018 /1,000,000*32/29 kg = 5.68×1012 kg
= 5.68 gigatonnes. Plus or minus a bit.I’m using this to do a mass balance on the atmosphere, ignoring lithosphere weathering.
Oxygen removed by CO2 burning – Oxygen depletion in the atmosphere = Net oxygen released by growing forests.
Which gives us a close estimate of net global biosphere growth every year. I hope.
Wouldn’t it be easier to look at the increase in CO2, rather the decrease in O2?
:-)
I was hoping you’d pick me up on that. CO2 leaves the atmosphere in two ways – to the biosphere and by solution in the oceans. The two ways are of almost equal magnitude.
For O2, the effect of O2 absorption or release by the oceans (abiologically) is negligible. That was an insight I picked up from the third IPCC report. I hadn’t realised it before then.
So for CO2, the equation is:
CO2 from burning – CO2 increase in the atmosphere = CO2 uptake by growing forests + CO2 uptake from oceans.Can’t get forest growth on its own without the mass balance on O2.
OK, sounds reasonable.
I’ll have to focus on the overpopulation thread then.
Slight problem, folks. I’m getting a negative biosphere growth from 1990 to 2017.
Or to put it another way, according to best available figures for CO2 emissions and atmospheric concentrations of oxygen, the world’s forests have been net absorbers of oxygen rather than net producers of oxygen.
The world’s fossil fuel burning from 1990 to 2017 has absorbed 571 gigatonnes of oxygen.
The amount of oxygen in the atmosphere has dropped by 635 gigatonnes.
So the world’s biosphere (mostly trees) has released -74 gigatonnes of oxygen.
I hope that’s not right.
mollwollfumble said:
Slight problem, folks. I’m getting a negative biosphere growth from 1990 to 2017.Or to put it another way, according to best available figures for CO2 emissions and atmospheric concentrations of oxygen, the world’s forests have been net absorbers of oxygen rather than net producers of oxygen.
The world’s fossil fuel burning from 1990 to 2017 has absorbed 571 gigatonnes of oxygen.
The amount of oxygen in the atmosphere has dropped by 635 gigatonnes.So the world’s biosphere (mostly trees) has released -74 gigatonnes of oxygen.
I hope that’s not right.
Simple mistake somewhere, presumably.
Bubblecar said:
mollwollfumble said:
Slight problem, folks. I’m getting a negative biosphere growth from 1990 to 2017.Or to put it another way, according to best available figures for CO2 emissions and atmospheric concentrations of oxygen, the world’s forests have been net absorbers of oxygen rather than net producers of oxygen.
The world’s fossil fuel burning from 1990 to 2017 has absorbed 571 gigatonnes of oxygen.
The amount of oxygen in the atmosphere has dropped by 635 gigatonnes.So the world’s biosphere (mostly trees) has released -74 gigatonnes of oxygen.
I hope that’s not right.
Simple mistake somewhere, presumably.
Maybe not.
There is a lot of forest clearance going on.
And then there are fires.
The Rev Dodgson said:
Bubblecar said:
mollwollfumble said:
Slight problem, folks. I’m getting a negative biosphere growth from 1990 to 2017.Or to put it another way, according to best available figures for CO2 emissions and atmospheric concentrations of oxygen, the world’s forests have been net absorbers of oxygen rather than net producers of oxygen.
The world’s fossil fuel burning from 1990 to 2017 has absorbed 571 gigatonnes of oxygen.
The amount of oxygen in the atmosphere has dropped by 635 gigatonnes.So the world’s biosphere (mostly trees) has released -74 gigatonnes of oxygen.
I hope that’s not right.
Simple mistake somewhere, presumably.
Maybe not.
There is a lot of forest clearance going on.
And then there are fires.
Ya, one of the three.
Have referred the matter to facebook, perhaps they can find the answer.
mollwollfumble said:
>>1 ppm in this case is 1 molecule of O2 in a million molecules of dry air. What’s that in gigatons of O2?> Is that what you wanted?
I didn’t express myself clearly.
1 ppm O2 is one O2 molecule in 1,000,000 molecules of air.
Mean mass of atmosphere is 5.148×1018 kg
Molecular weight of O2 is 32
Mean molecular weight of air molecule is 29.So mass of 1 ppm O2 is 5.148×1018 /1,000,000*32/29 kg = 5.68×1012 kg
= 5.68 gigatonnes. Plus or minus a bit.I’m using this to do a mass balance on the atmosphere, ignoring lithosphere weathering.
Oxygen removed by CO2 burning – Oxygen depletion in the atmosphere = Net oxygen released by growing forests.
Which gives us a close estimate of net global biosphere growth every year. I hope.
I think that 5.68 may be where i stuffed up. That could be ppm of oxygen rather than ppm of atmosphere. In which case i should have been using total mass of atmospheric oxygen rather than total mass of atmosphere.
Oxygen is 23.14% of the dry atmosphere by mass (20.95% by volume). Need to rethink.
mollwollfumble said:
mollwollfumble said:
>>1 ppm in this case is 1 molecule of O2 in a million molecules of dry air. What’s that in gigatons of O2?> Is that what you wanted?
I didn’t express myself clearly.
1 ppm O2 is one O2 molecule in 1,000,000 molecules of air.
Mean mass of atmosphere is 5.148×1018 kg
Molecular weight of O2 is 32
Mean molecular weight of air molecule is 29.So mass of 1 ppm O2 is 5.148×1018 /1,000,000*32/29 kg = 5.68×1012 kg
= 5.68 gigatonnes. Plus or minus a bit.I’m using this to do a mass balance on the atmosphere, ignoring lithosphere weathering.
Oxygen removed by CO2 burning – Oxygen depletion in the atmosphere = Net oxygen released by growing forests.
Which gives us a close estimate of net global biosphere growth every year. I hope.
I think that 5.68 may be where i stuffed up. That could be ppm of oxygen rather than ppm of atmosphere. In which case i should have been using total mass of atmospheric oxygen rather than total mass of atmosphere.
Oxygen is 23.14% of the dry atmosphere by mass (20.95% by volume). Need to rethink.
23.14% of 5.68 gigatonnes is 1.314 gigatonnes.
mollwollfumble said:
>>1 ppm in this case is 1 molecule of O2 in a million molecules of dry air. What’s that in gigatons of O2?> Is that what you wanted?
I didn’t express myself clearly.
1 ppm O2 is one O2 molecule in 1,000,000 molecules of air.
Mean mass of atmosphere is 5.148×1018 kg
Molecular weight of O2 is 32
Mean molecular weight of air molecule is 29.So mass of 1 ppm O2 is 5.148×1018 /1,000,000*32/29 kg = 5.68×1012 kg
= 5.68 gigatonnes. Plus or minus a bit.I’m using this to do a mass balance on the atmosphere, ignoring lithosphere weathering.
Oxygen removed by CO2 burning – Oxygen depletion in the atmosphere = Net oxygen released by growing forests.
Which gives us a close estimate of net global biosphere growth every year. I hope.
I think that 5.68 may be where i stuffed up. That could be ppm of oxygen rather than ppm of atmosphere. In which case i should have been using total mass of atmospheric oxygen rather than total mass of atmosphere.
Oxygen is 23.14% of the dry atmosphere by mass (20.95% by volume). Need to rethink.
23.14% of 5.68 gigatonnes is 1.314 gigatonnes.
I think it’s right now. Forest growth (as evidenced by net photosynthesis = photosynthesis – respiration) easily outstrips atmosphere changes. Human, other animal, fungi and plant respiration are all included in respiration. Forest products such as timber, paper and cotton are all included in forest growth.
Cumulative.
Per year.
Check units convert again.
1 per meg is one molecule of oxygen per million molecules of nitrogen.
Mean mass of atmosphere is 5.1480*10^18 kg = 5.148*10^6 gigatonnes.
Mass fraction of nitrogen in atmosphere is 75.72%
Mass ratio of oxygen to nitrogen is 8/7
So 1 per meg is 10^-6 * 5.148*10^6 * 0.7572 * 8/7 = 4.455 gigatonnes oxygen.
My calculation above was 4.8 (converting ppm to meg)
4.8 * 23.14% of 5.68 = 6.31 gigatonnes oxygen.
Dang it, still not a match.
mollwollfumble said:
mollwollfumble said:
>>1 ppm in this case is 1 molecule of O2 in a million molecules of dry air. What’s that in gigatons of O2?> Is that what you wanted?
I didn’t express myself clearly.
1 ppm O2 is one O2 molecule in 1,000,000 molecules of air.
Mean mass of atmosphere is 5.148×1018 kg
Molecular weight of O2 is 32
Mean molecular weight of air molecule is 29.So mass of 1 ppm O2 is 5.148×1018 /1,000,000*32/29 kg = 5.68×1012 kg
= 5.68 gigatonnes. Plus or minus a bit.I’m using this to do a mass balance on the atmosphere, ignoring lithosphere weathering.
Oxygen removed by CO2 burning – Oxygen depletion in the atmosphere = Net oxygen released by growing forests.
Which gives us a close estimate of net global biosphere growth every year. I hope.
I think that 5.68 may be where i stuffed up. That could be ppm of oxygen rather than ppm of atmosphere. In which case i should have been using total mass of atmospheric oxygen rather than total mass of atmosphere.
Oxygen is 23.14% of the dry atmosphere by mass (20.95% by volume). Need to rethink.
23.14% of 5.68 gigatonnes is 1.314 gigatonnes.
I think it’s right now. Forest growth (as evidenced by net photosynthesis = photosynthesis – respiration) easily outstrips atmosphere changes. Human, other animal, fungi and plant respiration are all included in respiration. Forest products such as timber, paper and cotton are all included in forest growth.
Cumulative.
Per year.
Dang it, I’ve done the O2 calculation three times now, three different ways, got three different answers, and I’m sure that all three are wrong.
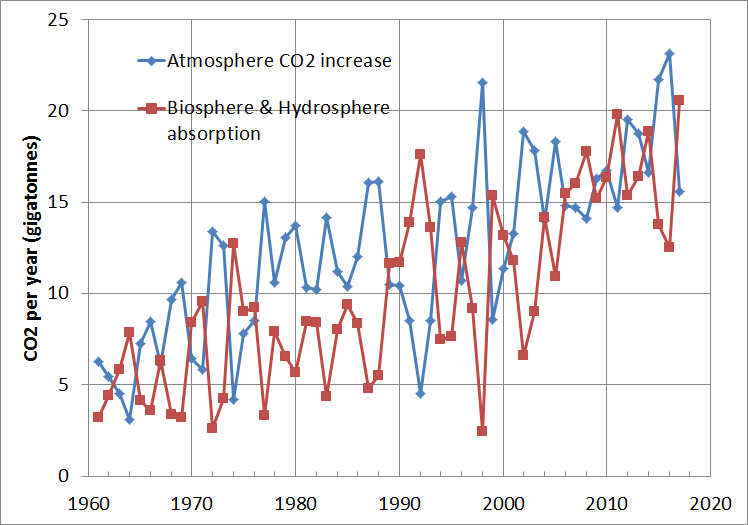
In the meantime, have a graph of CO2. The jaggedness is because of poor seasonal trend removal in the original Mauna Loa CO2 data. The opposite motion of the two graphs is because the atmospheric CO2 is subtracted from the smoothly varying total CO2 emissions to get the sum of biosphere and hydrosphere absorption.
The important point to note from this graph is that the uptake of CO2 by the biosphere and hydrosphere is only a little less than the increase of CO2 in the atmosphere.
https://www.sciencealert.com/nasa-images-show-just-how-much-carbon-monoxide-is-coming-off-the-burning-amazon?perpetual=yes&limitstart=1
Also note the NASA paper is ppmv (volume).
Michael V said:
https://www.sciencealert.com/nasa-images-show-just-how-much-carbon-monoxide-is-coming-off-the-burning-amazon?perpetual=yes&limitstart=1Also note the NASA paper is ppmv (volume).
“There’ll be bush-fires for sure, me man, There will, without a doubt; We’ll all be rooned, said Hanrahan, before the year is out.”
Well, parts per billion by volume, same diff.
By volume i think is the same as by mole, correct me if i’m wrong.
mollwollfumble said:
mollwollfumble said:
>>1 ppm in this case is 1 molecule of O2 in a million molecules of dry air. What’s that in gigatons of O2?> Is that what you wanted?
I didn’t express myself clearly.
1 ppm O2 is one O2 molecule in 1,000,000 molecules of air.
Mean mass of atmosphere is 5.148×1018 kg
Molecular weight of O2 is 32
Mean molecular weight of air molecule is 29.So mass of 1 ppm O2 is 5.148×1018 /1,000,000*32/29 kg = 5.68×1012 kg
= 5.68 gigatonnes. Plus or minus a bit.I’m using this to do a mass balance on the atmosphere, ignoring lithosphere weathering.
Oxygen removed by CO2 burning – Oxygen depletion in the atmosphere = Net oxygen released by growing forests.
Which gives us a close estimate of net global biosphere growth every year. I hope.
I think that 5.68 may be where i stuffed up. That could be ppm of oxygen rather than ppm of atmosphere. In which case i should have been using total mass of atmospheric oxygen rather than total mass of atmosphere.
Oxygen is 23.14% of the dry atmosphere by mass (20.95% by volume). Need to rethink.
23.14% of 5.68 gigatonnes is 1.314 gigatonnes.
I think it’s right now. Forest growth (as evidenced by net photosynthesis = photosynthesis – respiration) easily outstrips atmosphere changes. Human, other animal, fungi and plant respiration are all included in respiration. Forest products such as timber, paper and cotton are all included in forest growth.
Cumulative.
Per year.
Dang it, I’ve done the O2 calculation three times now, three different ways, got three different answers, and I’m sure that all three are wrong.
In the meantime, have a graph of CO2. The jaggedness is because of poor seasonal trend removal in the original Mauna Loa CO2 data. The opposite motion of the two graphs is because the atmospheric CO2 is subtracted from the smoothly varying total CO2 emissions to get the sum of biosphere and hydrosphere absorption.
The important point to note from this graph is that the uptake of CO2 by the biosphere and hydrosphere is only a little less than the increase of CO2 in the atmosphere.
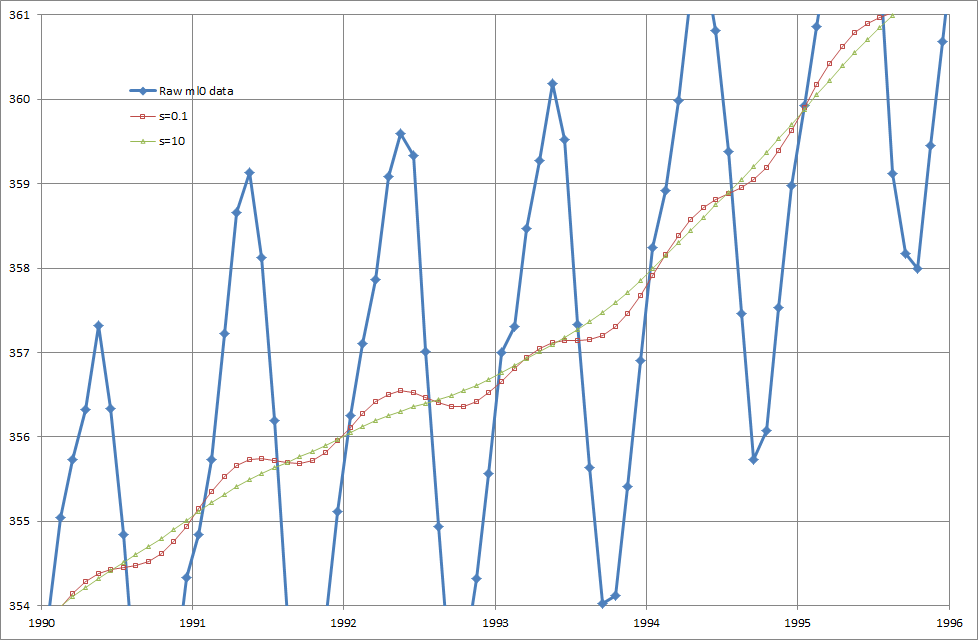
This is quite pretty. I’ve used the exact same smoothing algorithm, even to the same parameter s=10, that I used on buffy’s myopia data and applied it to CO2 data from Mauna Loa.
Here’s a detail of the result. The result (triangles) neatly removes the seasonal cycle from the Mauna Loa data without damaging the yearly trend. Don’t worry about whether this is a valid procedure, the Scripps people have already applied the same method to Mauna Loa’s O2 data.
mollwollfumble said:
>>1 ppm in this case is 1 molecule of O2 in a million molecules of dry air. What’s that in gigatons of O2?> Is that what you wanted?
I didn’t express myself clearly.
1 ppm O2 is one O2 molecule in 1,000,000 molecules of air.
Mean mass of atmosphere is 5.148×1018 kg
Molecular weight of O2 is 32
Mean molecular weight of air molecule is 29.So mass of 1 ppm O2 is 5.148×1018 /1,000,000*32/29 kg = 5.68×1012 kg
= 5.68 gigatonnes. Plus or minus a bit.I’m using this to do a mass balance on the atmosphere, ignoring lithosphere weathering.
Oxygen removed by CO2 burning – Oxygen depletion in the atmosphere = Net oxygen released by growing forests.
Which gives us a close estimate of net global biosphere growth every year. I hope.
Oh f^&*.
I’ve done the units convert four times now, and got four different answers. All four are wrong, because none is even in the correct ballpark.
The O2 concentration in the atmosphere is 20.95%
The Scripps conversion of 1 meg δO2/N2 = 1/0.2095 ppm = 4.8 ppm is wrong.
The correct conversion is 1 meg δO2/N2 = 1/(1-0.2095) ppm = 1.265 ppm.
But neither put me in the correct ballpark for gigatonnes of CO2.
Where’s a technical paper in which this stuff is done correctly?
mollwollfumble said:
The Scripps conversion of 1 meg δO2/N2 = 1/0.2095 ppm = 4.8 ppm is wrong.
4.8 per meg = 1ppm