Clean water is all around us, and more literally than you might think – it’s floating around in the air most of the time. Of course, it’s not particularly drinkable in that form, but now researchers at the Johns Hopkins Applied Physics Lab (APL) have found materials that can collect huge amounts of water from the air.
As is the case with similar systems we’ve covered previously, the key lies with a material called a metal-organic framework (MOF). These structures have the highest surface area of any known material – in fact, if you were able to unfold just one gram of an MOF, it would be enough to cover a football field. And all that internal space makes them perfect for capturing and storing water.
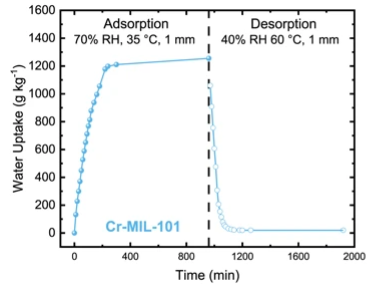
“We identified a MOF that could produce 8.66 liters (2.3 gall) of water per day per kilogram of MOF under ideal conditions, an extraordinary finding,” says Zhiyong Xia, co-lead author of the study. “This will help us deepen our understanding of these materials and guide the discovery of next-generation water-harvesting methods.”
https://newatlas.com/materials/harvester-drinking-water-thin-air/