https://arxiv.org/pdf/2007.00105
“Ancient Venus and Earth may have been similar in crucial ways for the development of life, such as liquid water oceans, land-ocean interfaces, the favorable chemical ingredients and energy pathways. If life ever developed on, or was transported to, early Venus from elsewhere, it might have thrived, expanded and survived the changes that have led an inhospitable surface on Venus today. Today the Venus cloud layer may provide a refugium for extant life. We introduce the Venus Life equation – a theory and evidence based approach to calculate the probability of extant life on Venus”.
A bit overoptimistic, I think. But fossils may not be out of the question.
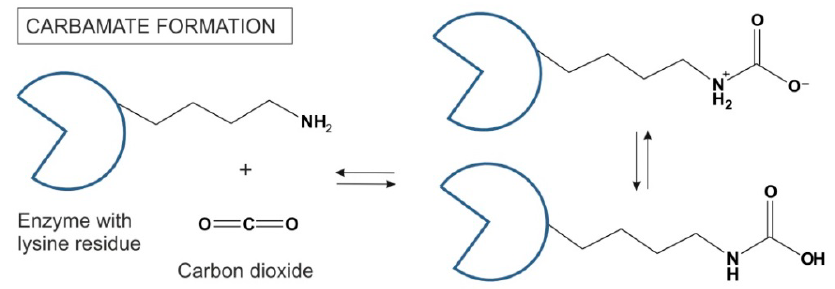
“A quick calculation using reported particle concentrations yields a count of 5×10^24 potential organisms suspended in the atmosphere. Other suggestions that have been put forward for Venus include surface life adapted to use supercritical carbon dioxide as a solvent (Budisa and Schulze-Makuch, 2014), and subsurface microbes in refugia of highly pressurized water (Schulze-Makuch et al., 2005).”
Having supercritical carbon dioxide solvent is a possibility that had never occurred to me.
https://www.mdpi.com/2075-1729/4/3/331/pdf
“Supercritical fluids have different properties compared to regular fluids and could play a role as life-sustaining solvents on other worlds. Even on Earth, some bacterial species have been shown to be tolerant to supercritical fluids. The special properties of supercritical fluids have recently been recognized in biotechnology and used to catalyze reactions that do not occur in water. One suitable example is enzymes when they are exposed to supercritical fluids such as supercritical carbon dioxide: enzymes become even more stable, because they are conformationally rigid in the dehydrated state. Planetary environments with supercritical fluids, particularly supercritical carbon dioxide, exist, even on Earth (below the ocean floor), on Venus, and likely on Super-Earth type exoplanets. These planetary environments may present a possible habitat for exotic life.”
“The change in properties from subcritical fluid to supercritical state is especially noteworthy for the common compounds water and carbon dioxide. These include:
(1) high solubility of gases within supercritical mixtures,
(2) miscibility of gases such as O2 and H2 in supercritical fluids,
(3) high diffusion rates and variable density, and
(4) high dissolving power.
As a conclusion, Ikushima advanced the case for supercritical fluids as an appropriate medium for chemical and biochemical processes under certain conditions”.
“Kalibanov was among the first to realize that the water bound to the enzyme determines the catalytic activity rather than the total water content of the system. The protein structure may largely be retained. For example, supercritical CO2 may dissolve from 0.3% to 0.5% (w/w) water, depending on the pressure and temperature. … Supercritical CO2 is believed to increase the fluidity of the cell membrane, enhancing its permeability and facilitate extraction of membrane components such as phospholipids”.
I find it startling that a water-based life form may exist in a CO2 solvent. I was expecting a different chemical system entirely.