Spiny Norman said:
So how much extra energy does it take to cool down the compressed air (which will be rather toasty thanks to the compression) down to -196° ?
I used to know that. Um, I don’t know it any more.
Specific heat of air at constant pressure is 1.006 kJ/kgK
Specific heat of atr at constant volume is 0.7171 kJ/kgK
But this isn’t constant pressure or constant volume.
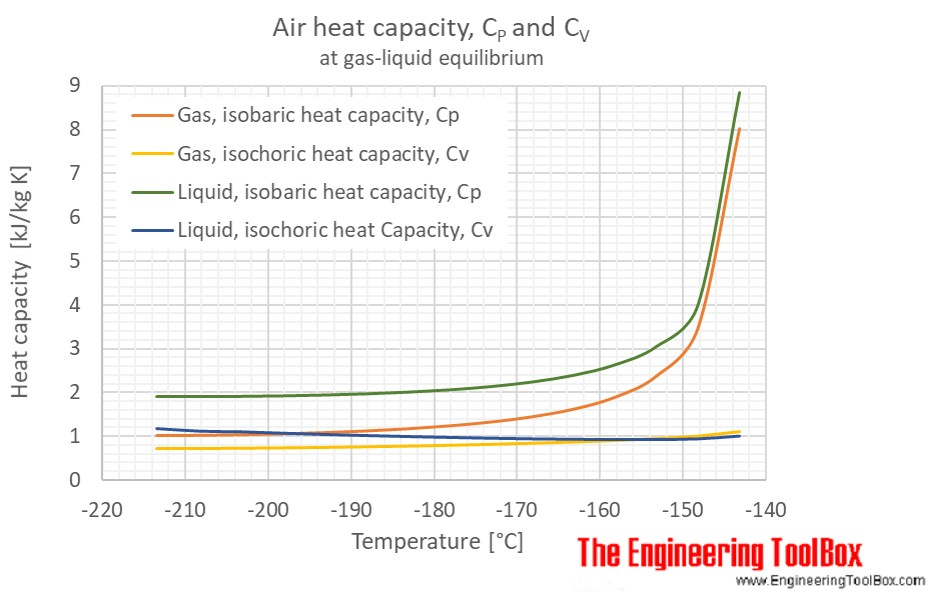
Perhaps this graph from Engineering Toolbox will help for a ballpark estimate.
From https://en.wikipedia.org/wiki/Cryogenic_energy_storage
In isolation the process is only 25% efficient.
But this is increased to around 50% when used with a low-grade cold store, such as a large gravel bed, to capture the cold generated by evaporating the cryogen. The cold is re-used during the next refrigeration cycle.
Efficiency is further increased when used in conjunction with a power plant or other source of low-grade heat that would otherwise be lost to the atmosphere. Highview Power claims an AC to AC round-trip efficiency of 70%, by using an otherwise waste heat source at 115 °C
Air can be liquefied by either the Linde Process or the Claude Process.
The Linde process is the only one I know. Air is alternately compressed, cooled, and expanded, each expansion results in a considerable reduction in temperature. With the lower temperature the molecules move more slowly and occupy less space, so the air changes phase to become liquid.
The Claude process. “The gas is allowed to expand isentropically twice in two chambers. While expanding, the gas has to do work as it is led through an expansion turbine. The gas is not yet liquid, since that would destroy the turbine. Commercial air liquefication plants bypass this problem by expanding the air at supercritical pressures. Final liquefaction takes place by isenthalpic expansion in a thermal expansion valve”.
