Tau.Neutrino said:
New quantum receiver the first to detect entire radio frequency spectrum
A new quantum sensor can analyze the full spectrum of radio frequency and real-world signals, unleashing new potentials for soldier communications, spectrum awareness and electronic warfare.
more…
How does this work? Wikipedia says:
A Rydberg atom is an excited atom with one or more electrons that have a very high principal quantum number, n. The higher the value of n, the farther the electron is from the nucleus, on average. Rydberg atoms have a number of peculiar properties including an exaggerated response to electric and magnetic fields, long decay periods and electron wavefunctions that approximate, under some conditions, classical orbits of electrons about the nuclei.
The arrival of tunable dye lasers in the 1970s allowed a much greater level of control over populations of excited atoms. In optical excitation, the incident photon is absorbed by the target atom, absolutely specifying the final state energy. The problem of producing single state, mono-energetic populations of Rydberg atoms thus becomes the somewhat simpler problem of precisely controlling the frequency of the laser output.
The large separation between the electron and ion-core in a Rydberg atom makes possible an extremely large electric dipole moment, d. There is an energy associated with the presence of an electric dipole in an electric field, F, known in atomic physics as a Stark shift.
I still don’t follow how it works. A Rydberg atom is unstable, both ways, it rapidly decays to lower energy states, and the slightest perturbation pushes it into ionisation.
Tau.Neutrino said:
More on the story
Scientists create quantum sensor that covers entire radio frequency spectrum
https://www.army.mil/article/212935
> Such wide spectral coverage by a single antenna is impossible with a traditional receiver system
Not necessarily. A sufficiently accurate spiral antenna has a very wide range of frequencies.
> While Rydberg atoms are known to be broadly sensitive, a quantitative description of the sensitivity over the entire operational range has never been done
Good point.
> To assess potential applications, Army scientists conducted an analysis of the Rydberg sensor’s sensitivity to oscillating electric fields over an enormous range of frequencies—from 0 to 1012 Hertz. The results show that the Rydberg sensor can reliably detect signals over the entire spectrum and compare favorably with other established electric field sensor technologies, such as electro-optic crystals and dipole antenna-coupled passive electronics.
Dipole! Could that be enough on its own with appropriate electronics?
> the Rydberg atoms’ exquisite sensitivity to electric fields
Hmm, possible.
> these atoms naturally operate over a very wide band of frequencies (from kilohertz (10^3 Hz) to terahertz (10^12 Hz)
Heck, that’s wide. I’m glad that said kilohertz instead of zero. There’s a lot of difference.
> For the ARL team, their atoms are held in a simple glass cell at room temperature, and they use two colours of laser light to simultaneously excite the Rydberg states and probe their reaction to the external electric fields
OK. That’s pretty standard.But I think it limits detection to scanning. The second laser scans through the frequency band.
Tau.Neutrino said:
Even more on the story.
Exciting apparatus helps atoms see the light
3-D trapping of Rydberg atoms in holographic optical bottle beam traps
Physicists show feasibility of building a trapped Rydberg ion quantum computer
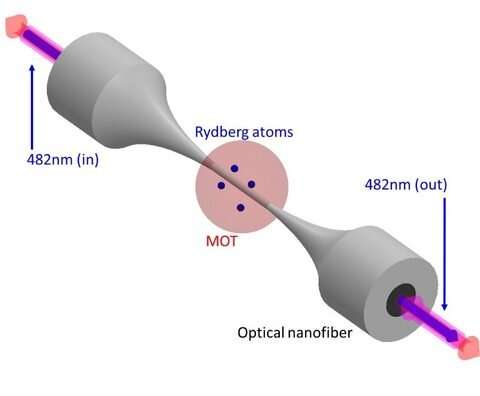
To carry out their research, the scientists used a device called a magneto-optical trap to capture a cluster of Rubidium (Rb) atoms. They reduced the temperature of the atoms to approximately 120 microKelvin—fractions of a degree above absolute zero and ran a nanofiber through the atom cloud.
Then, the scientists excited the Rb atoms to a more energetic Rydberg state, using a 482 nm beam of light travelling through the nanofiber.
What’s ‘n’ for 482 nm and rubidium?