I’ve never been fond of “habitable zone” defined as 273 to 373 Kelvin on the surface.
Because deeper can be hotter, and water is more stable at depth.
A new idea from an old SciFi book is to look for halogenated organic compounds as a sign of life in cold environments.
F2O closely resembles H2O in shape and polarity, and is liquid at a temperature of 49.3 to 128.4 Kelvin.
That’s exceedingly cold. Nitrogen melts at 63 Kelvin.
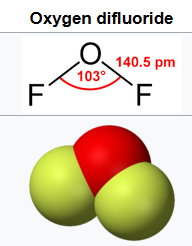
F2O is much more reactive than H2O, but it would need to be in order to operate at such low temperatures.
Can you see any reason why, in a cold environment with little free hydrogen, we wouldn’t see a carbon-based life form using fluorine instead of hydrogen? And thus have a habitable zone from 50 to 125 Kelvin. Just right for Titan, but a bit hot for Pluto.
Elements HCONSP replaced by FCONSP.
