Date: 20/07/2021 10:30:33
From: mollwollfumble
ID: 1767465
Subject: Who discovered the isotopes, and how?
(and I don’t mean Matt Groenig)
Date: 20/07/2021 11:37:11
From: SCIENCE
ID: 1767491
Subject: re: Who discovered the isotopes, and how?
the people who reliably computed atomic mass
Date: 20/07/2021 11:45:12
From: mollwollfumble
ID: 1767497
Subject: re: Who discovered the isotopes, and how?
SCIENCE said:
the people who reliably computed atomic mass
Who and how? I would very much like to know. Clearly there was some done by the time of Mendeleev, but even that early don’t know how they did it.
———
Otto Hahn and Lise Meitner for starters. Sometimes with Fritz Strassman.
Found many isotopes between lead and uranium
Later, catalogued the fission products of uranium and thorium.
Famous for early discoveries of 228Th (1905), 227Th, (1906), 228Ra, (1907), 230Th, (1907), Protactinium Pa, (1917)
Found that a single element may have multiple isotopes in 1921.
Between 1934 and 1938, Hahn, Meitner, and Strassmann found a great number of radioactive transmutation products.
Discovered rubidium-strontium dating and nuclear fission both in 1938.
By 1939 they had discovered 239U but not Neptunium.
———-
Irène Joliot-Curie found radioactive isotopes of N, Si and 30P in 1934, using alpha particle bombardment.
———-
But beyond that?
Date: 20/07/2021 11:52:54
From: SCIENCE
ID: 1767506
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
SCIENCE said:
the people who reliably computed atomic mass
Who and how? I would very much like to know. Clearly there was some done by the time of Mendeleev, but even that early don’t know how they did it.
———
Otto Hahn and Lise Meitner for starters. Sometimes with Fritz Strassman.
Found many isotopes between lead and uranium
Later, catalogued the fission products of uranium and thorium.
Famous for early discoveries of 228Th (1905), 227Th, (1906), 228Ra, (1907), 230Th, (1907), Protactinium Pa, (1917)
Found that a single element may have multiple isotopes in 1921.
Between 1934 and 1938, Hahn, Meitner, and Strassmann found a great number of radioactive transmutation products.
Discovered rubidium-strontium dating and nuclear fission both in 1938.
By 1939 they had discovered 239U but not Neptunium.
———-
Irène Joliot-Curie found radioactive isotopes of N, Si and 30P in 1934, using alpha particle bombardment.
———-
But beyond that?
but why does who does what matter, once it’s been done and the only thing they’re doing now is being dead
though since you mention a rather sex equal bunch of scientists there, we actually put the above objection on hold because we do argue that the advancement of females in STEM is worthy of note
Date: 20/07/2021 12:28:24
From: mollwollfumble
ID: 1767534
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
SCIENCE said:
the people who reliably computed atomic mass
Who and how? I would very much like to know. Clearly there was some done by the time of Mendeleev, but even that early don’t know how they did it.
———
Otto Hahn and Lise Meitner for starters. Sometimes with Fritz Strassman.
Found many isotopes between lead and uranium
Later, catalogued the fission products of uranium and thorium.
Famous for early discoveries of 228Th (1905), 227Th, (1906), 228Ra, (1907), 230Th, (1907), Protactinium Pa, (1917)
Found that a single element may have multiple isotopes in 1921.
Between 1934 and 1938, Hahn, Meitner, and Strassmann found a great number of radioactive transmutation products.
Discovered rubidium-strontium dating and nuclear fission both in 1938.
By 1939 they had discovered 239U but not Neptunium.
———-
Irène Joliot-Curie found radioactive isotopes of N, Si and 30P in 1934, using alpha particle bombardment.
———-
But beyond that?
There were charts of known isotopes/nuclides in all three years 1948, 1949, 1950 but I don’t see them on the web. The 1949 chart is called “Trilinear chart of nuclear species”.
By 1960, “over 1300 different nuclides are presently known”.
In wikipedia now, “beyond the 339 naturally occurring nuclides, more than 3000 radionuclides of varying half-lives have been artificially produced and characterized.”
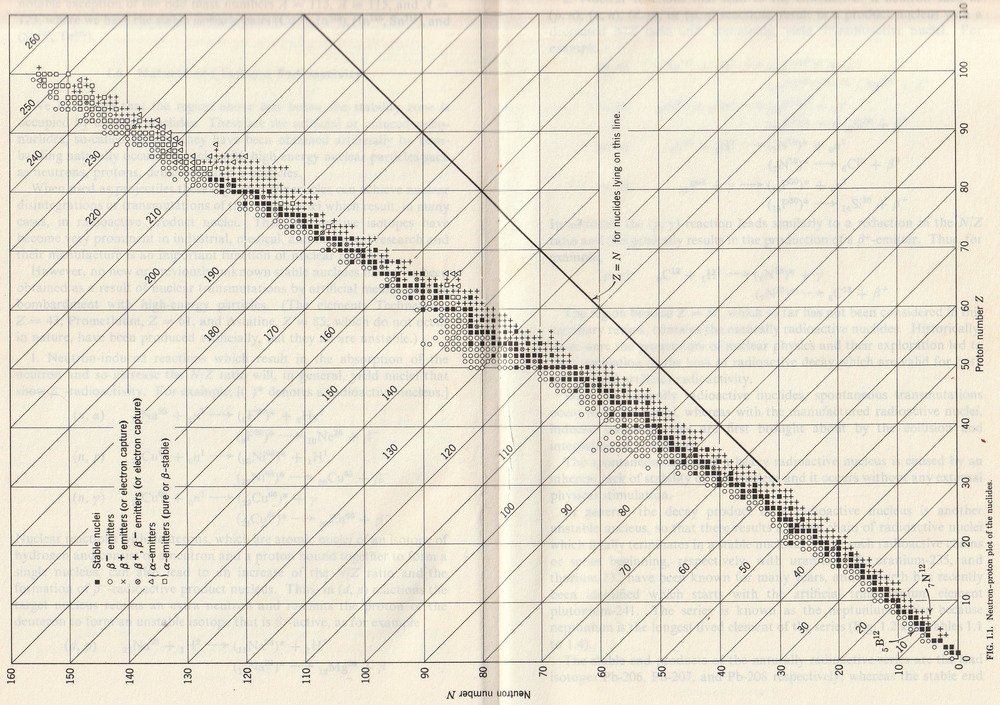
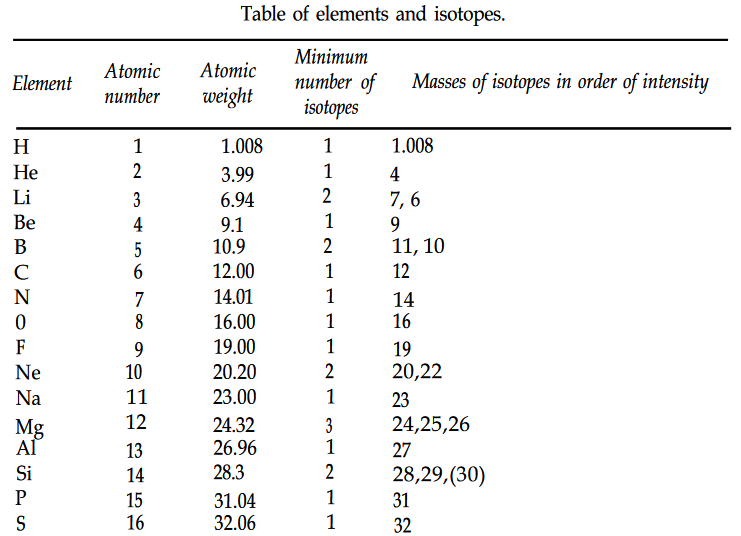
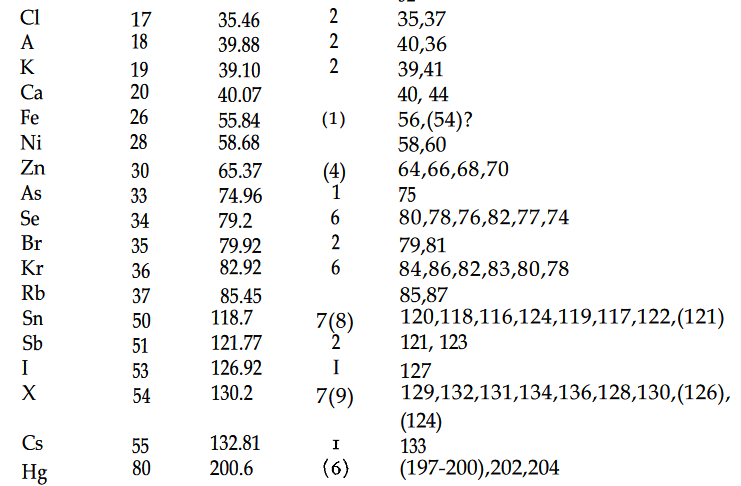
The Table of isotopes from 1960 is shown below. Note how it stops at element 101.
Also note how there are were 6 known isotopes of oxygen, compare that with the 16 isotopes of oxygen now known.
This still doesn’t solve the questions of who and how.
Date: 20/07/2021 13:37:26
From: mollwollfumble
ID: 1767585
Subject: re: Who discovered the isotopes, and how?
Aha. Typing this now before I lose the reference.
https://sci-hub.st/https://journals.aps.org/rmp/abstract/10.1103/RevModPhys.16.1
Published 1944 (before the end of WWII you’ll notice) by Glenn T Seaborg as “Table of isotopes” in Rev. Mod. Phys.
Seaborg was then working at the Department of Chemistry, University of California,Berkeley,
This is not that long after the earliy pioneers of Hahn, Joliot-Curie etc.
The table, amusingly and instructively, gives each isotope an authenticity rating called “class” from A (mass number and element certain) to G (probably in error). I’ve seen similar ratings given for subatomic particle tables. The authenticity rating is a strategy that has fallen out of favour, but needs to be urgently revived for modern use.
The largest element in the table is 93, neptunium, but not the most stable isotope of neptunium. That makes sense and has relevance in finding islands of stability. It is usually easier to find highly radioactive isotopes than stable ones because highly radioactive ones shout their existence even when there are only a few atoms present. There are 5 isotopes of oxygen, as against 6 in 1960 and 16 now.
The “how” is briefly listed by the nuclear reaction by which it was found. And there are literally hundreds of people listed as authors in the references associated with the discoveries of these isotopes. Most references are from the 1930s, not surprisingly.
The naturally occurring isotopes are not given a rating, but are given a reference. The reference, though, would be to the most accurate calculation of natural abundance rather than the discovery date. eg. Deuterium was discovered before the referenced paper which was published (1936), it was discovered and named in 1931 by Harold Urey.
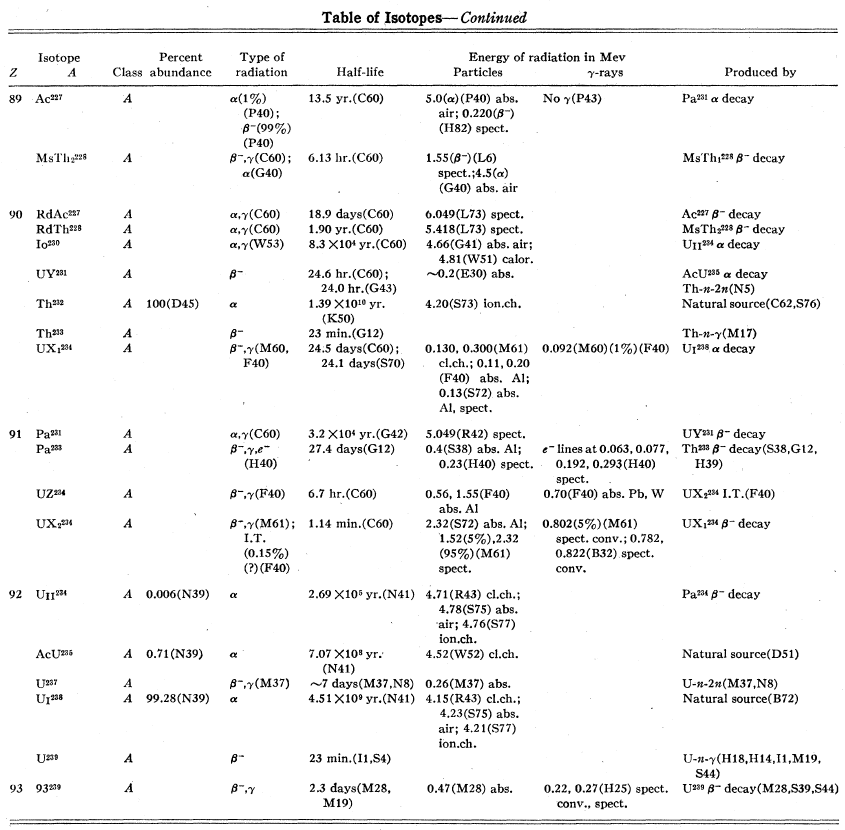
Start of table, remember that this is as known in 1944.

End of table. Note that C60 does not refer to cobalt-60, it refers to the 60th reference by an author whose name starts with C, in this case a summary paper in 1931 by Curie, Debierne, Eve, Geiger, Hahn, Lind, St. Meyer, Rutherford, and Schweidler.
(call it Schweidler’s list)
Date: 20/07/2021 15:25:06
From: mollwollfumble
ID: 1767632
Subject: re: Who discovered the isotopes, and how?
The golden age for the discovery of isotopes was definitely after 1935, whith literally many hundreds of papers and authors between 1935 and 1944. This was also an age when most papers had only one author, having two or more authors was definitely the exception rater than the rule.
In a summary by Curie and others the only radioactive isotopes known in 1931 were the alpha decay chains of Uranium, Thorium, Actinium and Radium.
Discoverers prior to 1935 are so rare that I can actually list them and sort them by date.
1896 – Becquerel
1898 – Curie
1898 – Schmidt – I’d like to know more about this one!
1906 – Campbell & Wood
1922 – Geiger
1923 – Meitner
1928 – Kolhorster
1930 – V Grosse
1931 -
1932 – Gueben, Gratias, Collie, Hevesy, Pahl
1933 – Libby, Latimer, Ornstein, Vreeswijk
1934 – Aston, Crane, Juliot, Frisch, McKellar, Vaughan, Williams, Tate
Other early researchers (not necessarily discoverers) are:
Dangit, too many to list right now. Let’s look pre-1920. All of these worked on the decay chains of Ac, U, Th, Ra.
1908 – O. Hahn, L. Meitner
1909 – F.v.Lerch, E.v.Lartburg
1911 – H.G.T.Moseley, K.Fajans, A.F.Kovarik
1913 – H.N.McCoy
1914 – P.B.Perkins, St Meyer, F.Paneth, V.F.Hess
1917 – R.Schmid, B.Walter
1919 – F.Soddy
1919 – E.Albrecht
Date: 20/07/2021 19:59:09
From: mollwollfumble
ID: 1767710
Subject: re: Who discovered the isotopes, and how?
Adding up number of isotopes known.
Somewhere between 550 and 820 isotopes in 1944.
More than 1300 isotopes in 1960.
More than 3400 isotopes now.
Lots of famous names involved in the discovery of isotopes.
Even the Governor of South Australia – Sir Mark Oliphant. Famous for the discovery of Tritium, Helium-3 and nuclear fusion. And for his book about Rutherford.
Do you think we could petition for an isotope named Oliphantium?
Date: 21/07/2021 10:28:18
From: mollwollfumble
ID: 1767944
Subject: re: Who discovered the isotopes, and how?
> 1896 – Becquerel
> 1898 – Curie
> 1898 – Schmidt – I’d like to know more about this one!
Got it. Schmidt and Wiedermann are independently credited with the discovery of 232Th in 1898.
I bet you didn’t know that.
> 1906 – Campbell & Wood
Campbell & Wood discovered 37Rb in 1906. This is a radioactive isotope that makes up 27% of natural rubidium.
> 1908 – O. Hahn, L. Meitner
> 1909 – F. v. Lerch, E. v. Lartburg
van Lerch & van Lartberg and Hahn & Meitner independently discovered 208Tl in 1909.
> 1914 – P.B.Perkins, St Meyer, F.Paneth, V.F.Hess
> 1922 – Geiger
Geiger didn’t discover a new isotope. He measured the particle energies from 230Th in 1922. This isotope had been discovered by St Meyer in 1916.
Date: 21/07/2021 12:00:29
From: mollwollfumble
ID: 1768011
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
> 1896 – Becquerel
> 1898 – Curie
> 1898 – Schmidt – I’d like to know more about this one!
Got it. Schmidt and Wiedermann are independently credited with the discovery of 232Th in 1898.
I bet you didn’t know that.
> 1906 – Campbell & Wood
Campbell & Wood discovered 37Rb in 1906. This is a radioactive isotope that makes up 27% of natural rubidium.
> 1908 – O. Hahn, L. Meitner
> 1909 – F. v. Lerch, E. v. Lartburg
van Lerch & van Lartberg and Hahn & Meitner independently discovered 208Tl in 1909.
> 1914 – P.B.Perkins, St Meyer, F.Paneth, V.F.Hess
> 1922 – Geiger
Geiger didn’t discover a new isotope. He measured the particle energies from 230Th in 1922. This isotope had been discovered by St Meyer in 1916.
Whoa, I was about to make a big mistake, saying that 210Pb, also known as Radium D, wasn’t discovered until 1929. And dozens of other similar discovery mistakes.
When I accidentally found an article from January 1909, that lists almost all the intermediate radioactive decay products from three of the four decay chains, from Uranium (including Radium), Thorium and Actinium. Missing only the Neptunium decay chain. So Radium D for example was known by Jan 1909. That is early, only eleven years after M. Curie’s pioneering work.
Here’s the reference. https://hal.archives-ouvertes.fr/jpa-00242321/document

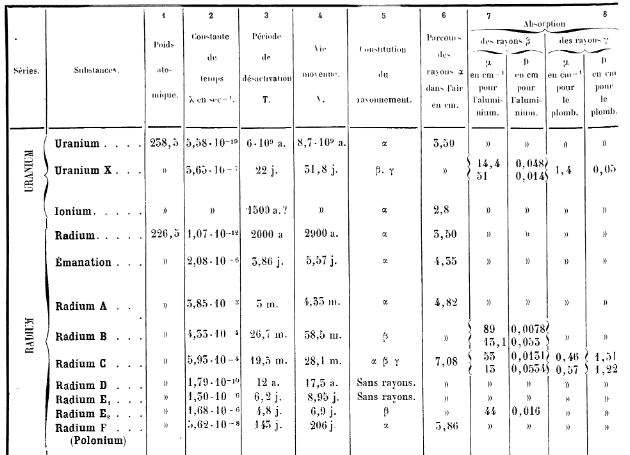
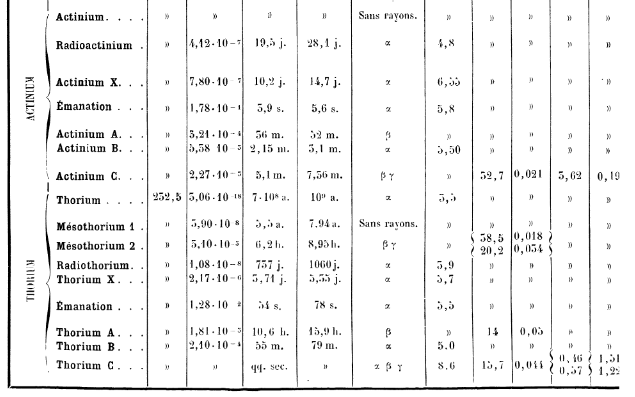
Where did Actinium come from? Specifically the isotope of actinium at the head of the actinium decay chain. Ah, I see, that is actually the 235U decay chain, so is found naturally in pitchblende.
This is amusing, https://en.wikipedia.org/wiki/Actinium#History
There is debate over whether actinium was discovered in 1899 or 1902. “Hindsight criticism of the early publications should be mitigated by the then nascent state of radiochemistry. Highlighting the prudence of Debierne’s claims in the original 1899 papers, nobody can contend that Debierne’s substance did not contain actinium. Debierne lost interest in the element and left the topic. Giesel (1902), on the other hand, can rightfully be credited with the first preparation of radiochemically pure actinium and with the identification of its atomic number 89.”
Date: 21/07/2021 16:26:56
From: mollwollfumble
ID: 1768118
Subject: re: Who discovered the isotopes, and how?
OK, so where am I?
(Dates are publication dates, rather than discovery dates).
- Starting with the discovery of the radioactivity of Uranium by Becquerel in 1896.
- Next the discovery of Radium and Polonium among uranium ores by Curie in 1898.
- At the same time the discovery of the radioactivity of Thorium by Schmidt in 1898.
- Actinium was found in uranium ores in 1899 by Debierne, and characterised by Giesel in 1902.
- By 1909, the major comonents of all three decay chains of 238U, 232Th and 235U (ie Actinium) had been found.
- Going back to 1905, J.J. Thomson found radioactivity in Rubidium and Potassium. This was clarified in 1906 by others.
…
- Starting in 1935, there were hundreds of science papers about isotope discovery and characterisation.
- In 1939, Otto Hahn and Lise Meitner found nuclear fission of Uranium. Nuclear fission of Thorium the same year.
- By early in 1940, more than 100 science articles had been written about nuclear fission, and a few fission products had been found.
- By 1944, about 550 isotopes were reliably characterised, with a further 300 tentative isotope discoveries.
- By 1960, more than 1300 isotopes were reliably characterised.
One method of producing slow neutrons in 1939 was using a cyclotron with deuterium, The dueterium ions hit lithium to produce neutrons, which were slowed by a block of paraffin to slow neutrons, which induced fission in uranium and thorium.
Isotope discovery resembles a cottage industry. With each person working separately on this exciting frontier of physics, being coordinated and helped by some famous “experts” in the field. Though the experts more than anything just had the luck of being around in the earliest stages.
Date: 21/07/2021 17:06:04
From: dv
ID: 1768132
Subject: re: Who discovered the isotopes, and how?
There are about 3000 known nuclides so you might have a bit of work to do
Date: 22/07/2021 06:26:22
From: mollwollfumble
ID: 1768319
Subject: re: Who discovered the isotopes, and how?
dv said:
There are about 3000 known nuclides so you might have a bit of work to do
Start at the beginning, go to the end and then stop. :-) I’m hoping that later discoveries were made by “big science” labs, which greatly reduces the number of discoverers and methods.
———
Keeping in mind that Bequerel’s discovery of the radioactivity of Uranium was in 1896.
Neon was discovered in 1894 by Lord Rayleigh. He happened to notice that the atomic weight of nitrogen from the air did not agree with the atomic weight of nitrogen from the destruction of nitrogen-containing compounds, and correctly deduced that the air contained another gas that was extremely unreactive. He calculated the atomic weight of Neon at 19.09, not too bad for a preliminary estimate, though most other elements already atomic weights accurate within 0.01%.
This threw the entire periodic table into disarray for a very brief time as the column for noble gases was added.
With the exception of the noble gases, atomic weights were just about sorted (72 elements) before the year 1894. Most of what happened later was refinement. This came in useful later in calculating binding energies. The whole principle of finding atomic weights came from molecules. A molecule contains atoms in a fixed ratio. By removing one element we calculate the difference in weight and the ratio of that to the weight of the original molecule gives the atomic weight. The first method used was called the “gravimetric method”. Shortly before 1894, another method, the electrolytic method, was invented. A pure element was deposited on an electrode made of platinum, then reacted with another element such as iodine and the change in weight measured. From the known atomic weight of iodine, the atomic weight of the unknown was calculated.
I’m discussing this in detail for two reasons. One is that this work would eventually lead to the calculation of binding energies. A second is that the calculation of atomic weights led to the eventual realisation that shable elements had more than one isotope. Already by 1894, there were problems with the atomic weight ratio of Hydrogen to Oxygen, the atomic weight of Palladium, and most troublingly the atomic weight of Tungsten. No one at the time twigged to the fact that these problems were due to the differing isotope ratios in the samples.
———-
The realisation that what had been believed to be transuranic elements were actually fission fragments came in 1939 with Otto Hahn. Hahn’s partner Lise Meitner was not originally convinced but very soon realised that Hahn was right. By the end of the year 1939, at least 37 radioactive fission fragments had been identified, that were isotopes of 13 elements. Fission had been found for Uranium, Thorium, and Protactinium.
There were two absolutely fundamental methods used in discovering these radioactive isotopes. Both quite clever.
The first was to mix the fission products with a known element, such as Barium. Then chemically purify the Barium. The radioactivity that was uniformly distributed throughout the purified Barium belongs to isotopes of Barium among the fission fragments. In this way the fission fragments were separated by element. The further separation was by half life and decay chains. By the end of 1939, seven radioactive isotopes of Tellurium had been found this way, although only three had known atomic masses.
The second fundamental method was to use the kinetic energy of fission fragments to sort them out. An element that absorbs a neutron to get bigger or to decay in the normal way by alpha and beta decay does not have a large kinetic energy so remains in the original sample. At least two new isotopes were found this way. But fission products are ejected from the original sample because of their large kinetic energy so can be collected separately on some surface such as a water, glass or metal surface. Then the result was dissolved. This is an instant separation out of all fission fragments from the original sample. Nice. This process was invented by Meitner and Fisch (1939).
Date: 22/07/2021 09:49:26
From: mollwollfumble
ID: 1768372
Subject: re: Who discovered the isotopes, and how?
Dang it. Lost post. Try again.
All the isotopes of all the stable elements were known by 1942.
Several methods were used, such as the speed of diffusion, speed of electrolysis, and mass spectroscopy.
Urey got the Nobel prize in 1934 for the discovery of deuterium. It was known in 1931 that deuterium had to exist.
This is the situation for isotopes of stable elements in 1922, from the Nobel Lecture by F. W. Aston.
Mass Spectroscopy.
“In the parabola method of analysis devised by Sir J. J. Thomson, the rays generated by means of an electric discharge, after reaching the surface of the cathode, enter a long and very fine metal tube. By this means a narrow beam of rays is produced which is subjected to deflection by electric and magnetic fields, and finally falls upon a screen of fluorescent material or a photographic plate.”
“Wilhelm Wien found that strong electric or magnetic fields deflected the canal rays and, in 1899, constructed a device with perpendicular electric and magnetic fields that separated the positive rays according to their charge-to-mass ratio (Q/m). Wien found that the charge-to-mass ratio depended on the nature of the gas in the discharge tube. English scientist J. J. Thomson later improved on the work of Wien by reducing the pressure to create the mass spectrograph.”
The first work on mass spectroscopy was done on gases.
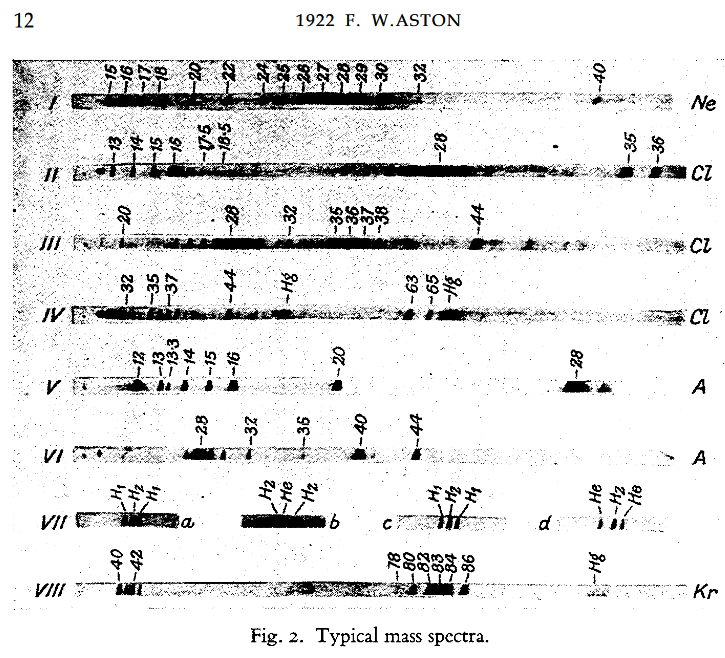
Date: 22/07/2021 15:04:40
From: mollwollfumble
ID: 1768562
Subject: re: Who discovered the isotopes, and how?
I unearthed my father’s “handbook of chemistry and physics 1937/38 edition”, which has a list of isotopes.
The date is critical because it is before Hahn’s discovery of nuclear fission (1939).
It doesn’t have any other isotopes dollowing on from Irene Juliot-Curie’s discovery in 1934 of artificially induced radiactivity. It does have the Julion-Curie isotope of 17O, but has it listed as a natural isotope of oxygen.
The HCP list accidentally omits the natural radioactive isotope 40K (the banana isotope) discovered circa 1906, I think.
So all the isotopes listed in the HCP 1937/38 edition can be called “natural”. Mostly from the lists prepared of stable isotopes by F. W. Aston.
Adding up number of isotopes known.
About 300 isotopes in 1937
> Somewhere between 550 and 820 isotopes in 1944.
> More than 1300 isotopes in 1960.
> More than 3400 isotopes now.
Of the isotopes in HCP 1937/38, I want to look more closely into the radioactive isotopes of Lead and above. Because my previous reference for these was back in 1906.
- There are 8 naturally occurring isotopes of Lead. Three of these are “radiogenically derived”
- 206Pb, 207Pb, 208Pb = radiogenically derived stable isotopes
- 203Pb, 204Pb, 205Pb, 209Pb, 210Pb = other stable natural isotopes
- 201Pb, 202Pb, 211Pb, 213Pb, 214Pb, 215Pb, 216Pb = other isotopes known in 1933
How do these other isotopes of lead tie in with decay chains?
Looking up wikipedia for lead isotopes in decay chains.
- Pb206, Pb207, Pb208, Pb209, Pb210, Pb,211, Pb212, Pb214
- That leaves 201Pb, 202Pb, 213Pb, 215Pb & 216Pb extra, unaccounted for
I’ll need to look up the reference Bishop et al. (1933) to find out how they were discovered. These have half lives go down to 1 minute.
For Uranium in HCP 1937/38, the following isotopes are known.
- 233U, 234U, 235U, 236U, 237U, 238U, 239U, 240U = isotopes known in 1933
- The main isotopes of uranium from wikipedia are 233U, 234U, 235U, 236U, 238U, which leaves three isotopes unaccounted for.
The reference here is Goslin et al. (1933).
Lead Isotopes
Edna R. Bishop, Margaret Lawrenz, and C. B. Dollins
Phys. Rev. 43, 43 – Published 1 January 1933 (received Oct 1932)
(Edna and Margaret are discoverers, just to please the sexist among you)
“The magneto-optic method shows that lead has sixteen isotopes, found in C.P. uranium and thorium salts. The differential time lag varies directly with the weight of the isotopes of a given element. The order of abundance of isotopes is also given by the amount of rotation of the analyzing nicol necessary to extinguish their minima.”
This “magneto-optic” method was described in 1930.
“Monochromatic light passes successively through a Nicol prism, two cylindrical glass cells containing the sample, and a second Nicol prism. The two glass cells are placed coaxially inside two cylindrical tubes around which helices of wire have been wound. If the same substance is placed in the two cells and the electric circuit so adjusted that the magnetic fields in the two cells are equal and opposite and are applied simultaneously then no light passes through the second Nicol prism. etc.” OK, a bit weird, but based on the use of a similar method in analysing crystal minerals in rocks.
The Isotopes of Uranium, Thorium and Thallium
Roy Goslin and Fred Allison
Phys. Rev. 43, 49 – Published 1 January 1933, (received Oct 1932)
The same “magneto-optic” method was used.
Date: 22/07/2021 18:41:56
From: mollwollfumble
ID: 1768693
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
This “magneto-optic” method was described in 1930.
“Monochromatic light passes successively through a Nicol prism, two cylindrical glass cells containing the sample, and a second Nicol prism. The two glass cells are placed coaxially inside two cylindrical tubes around which helices of wire have been wound. If the same substance is placed in the two cells and the electric circuit so adjusted that the magnetic fields in the two cells are equal and opposite and are applied simultaneously then no light passes through the second Nicol prism. etc.” OK, a bit weird, but based on the use of a similar method in analysing crystal minerals in rocks.
I can’t help wondering if this magneto-optic method from 1930 is a direct precursor of NMR spectroscopy, supposeldly invented later, in 1944.
So far as I can tell, the magneto-optic method uses electromagnetism (from an electromagnet) to excite the spin in the nucleus of an isotope, which is read out as a polatisation of light passing through the medium containing that isotope.
In the magneto-optic method the input is a time-varying magnetic field in a coil of wire and the output is a change in the polarisation of visible right. In NMR spectroscopy the input is radio waves and the output is a change in the radio signal. But apart from a change in frequency from optical to radio wavelength for the output and change in waveform for the inlet, the two appear identical.
(next step, look at how the Julioty-Curie method of producing new radioactive isotopes in 1934 affected the discovery of isotopes ten years later).
Date: 22/07/2021 21:15:12
From: mollwollfumble
ID: 1768744
Subject: re: Who discovered the isotopes, and how?
Hold on a moment.
> Of the isotopes in HCP 1937/38, I want to look more closely into the radioactive isotopes of Lead and above.
> 201Pb, 202Pb, 211Pb, 213Pb, 214Pb, 215Pb, 216Pb = other isotopes known in 1932/33
> That leaves 201Pb, 202Pb, 213Pb, 215Pb & 216Pb extra, unaccounted for
Check that against isotopes known in Seaborg’s table in 1944.
201Pb, 202Pb, 213Pb, 215Pb & 216Pb were known in 1932/33 but not in 1944. Either they are accidentally missing from Seaborg’s table (Like 40K is missing from the HCP 1937/38 table) or claims of their existence were rejected. Neither option seens likely as Seaborg was very thorough.
> For Uranium in HCP 1937/38, the following isotopes are known.
> 233U, 234U, 235U, 236U, 237U, 238U, 239U, 240U = isotopes known in 1932/33
> The main isotopes of uranium from wikipedia are 233U, 234U, 235U, 236U, 238U, which leaves three isotopes unaccounted for.
Check that against isotopes known in Seaborg’s table in 1944.
240U was known in 1932/33 but not in 1944.
These discrepancies need checking, but not by me.
————-
As for the influence of Joliot-Curie’s discovery of making artificial radioactive isotopes in 1934, more than half of the radioactive isotopes known in 1944 were produced in much the same way. ie. by bombarding an element with either a gamma ray, fast neutron, slow neutron, proton, deuteron or alpha particle.
Having the same isotope able to be produced by 5 or more different ways is not at all unusual.
eg. the lightest isotope of Titanium known in 1944 was 45Ti. It could be produced in five ways.
- Bombarding Titanium with a gamma ray
- ” Titanium with a neutron
- ” Scandium with a deuteron
- ” Scandium with a proton
- ” Calcium with an alpha particle
Date: 23/07/2021 06:02:53
From: mollwollfumble
ID: 1768861
Subject: re: Who discovered the isotopes, and how?
Is that all there is? Is it the case that all new isotopes after 1942, the end of the stable isotopes, were produced by firing nanoscale bullets (gamma, n, H, D, He) at elements?
It can’t be true, can it. Let’s look.
For starters, “Einsteinium was discovered as a component of the debris of the first hydrogen bomb explosion in 1952.” This explosion is “Ivy Mike”.

This paper refers back to

Which I’ll save for later.
Papers on “Mike” go back to 1955.
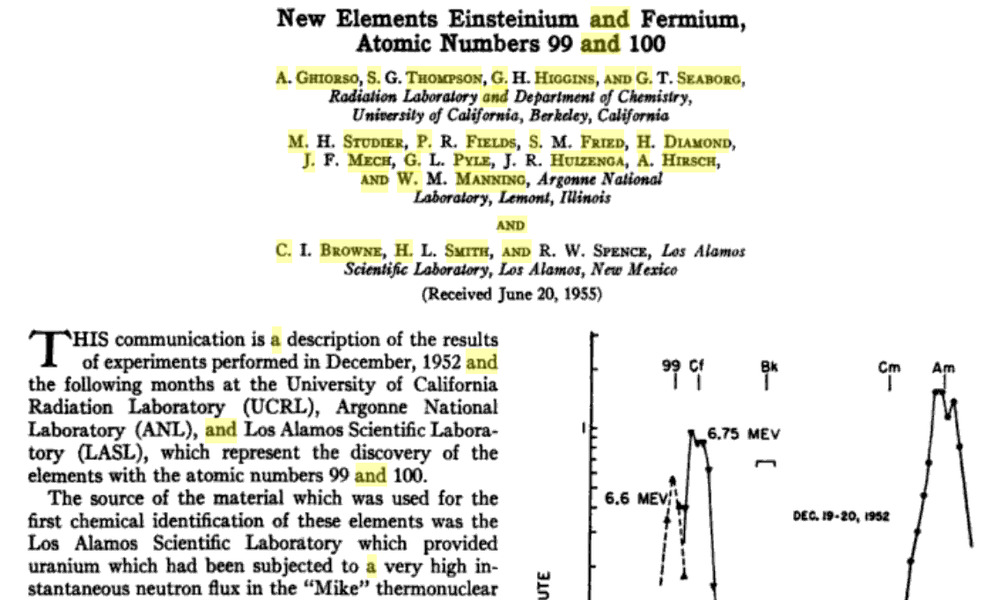
Methods

Results
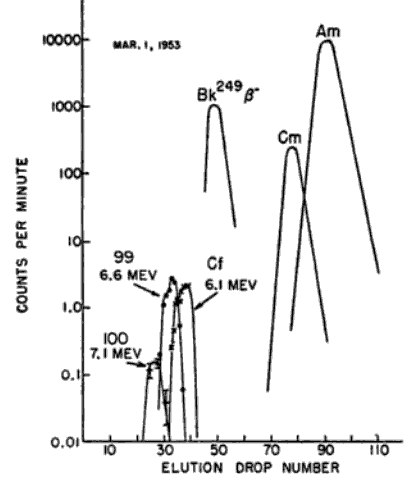
Returning to the paper from 1960,
Because of the high neutron flux, isotopes of Uranium were produced with masses of 239 to 255, and these decayed to nuclides with both higher (beta decay) and lower (alpha decay) atomic number.
From 1960 we have this figure. Note the half lives for some of the beta decays detected.
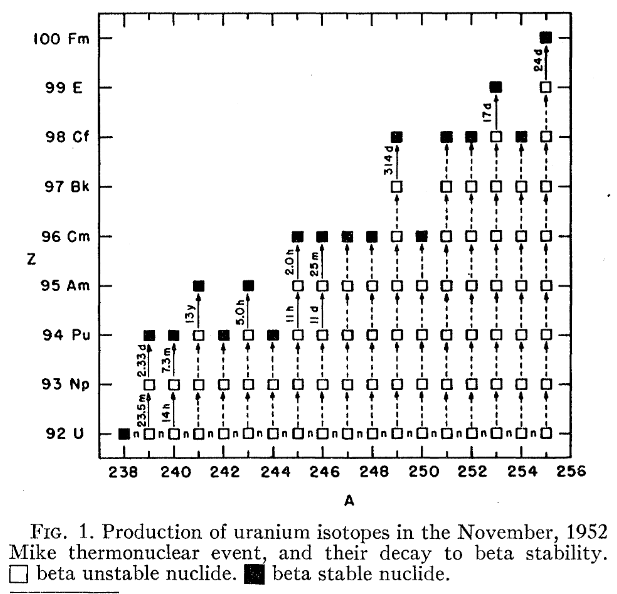
I see from the following that the use of mass spectrometry is not limited to the stable isotopes.
Date: 23/07/2021 20:38:34
From: mollwollfumble
ID: 1769382
Subject: re: Who discovered the isotopes, and how?
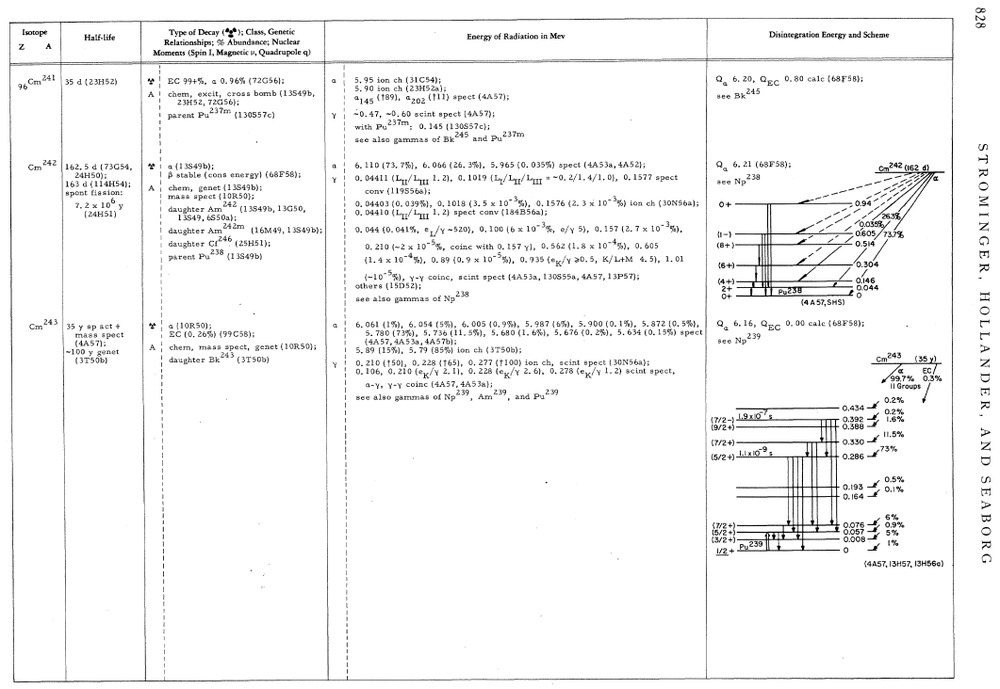
Still reading the 1958 summary of Isotopes known at that time.
Fascinating reading. For each isotope it has the nuclear energy levels of the isotope and its decay product.
Forget about “metastable” nuclides, nucleus energy levels are far more complicated than that.
Did you know that nuclei sometimes naturally jump up to a higher energy level? I hadn’t.
Another unexpected bonus from the table is the discovery of Californium in the spectrum of a distant supernova.
By 1958, all elements were known up to element 102, discovered by a Swedish group, not yet named Nobellium.
Nobel – Sweden, get it?
A typical page, showing energy levels for some isotopes of Curium. The last 2 digits of the reference number gives the date of discovery. eg. 13S49b was published in 1949.
Date: 24/07/2021 03:16:25
From: mollwollfumble
ID: 1769526
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
Still reading the 1958 summary of Isotopes known at that time.
Did you know that nuclei sometimes naturally jump up to a higher energy level? I hadn’t.
Not quite “naturally”, by “Coulomb excitation”.
Wiki says: “Coulomb excitation is a technique in experimental nuclear physics to probe the electromagnetic aspect of nuclear structure. In coulomb excitation, a nucleus is excited by an inelastic collision with another nucleus through the electromagnetic interaction. In order to ensure that the interaction is electromagnetic in nature — and not nuclear — a safe scattering angle is chosen.”
Not quite pertinent to the thread, because it discovers new enegy levels in nuclides rather than new nuclides, but interesting nonetheless.
Date: 24/07/2021 04:09:08
From: mollwollfumble
ID: 1769528
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
mollwollfumble said:
Still reading the 1958 summary of Isotopes known at that time.
Did you know that nuclei sometimes naturally jump up to a higher energy level? I hadn’t.
Not “naturally”, by “Coulomb excitation”.
Wiki says: “Coulomb excitation is a technique in experimental nuclear physics to probe the electromagnetic aspect of nuclear structure. In coulomb excitation, a nucleus is excited by an inelastic collision with another nucleus through the electromagnetic interaction. In order to ensure that the interaction is electromagnetic in nature — and not nuclear — a safe scattering angle is chosen.”
Not quite pertinent to the thread, because it discovers new enegy levels in nuclides rather than new nuclides, but interesting nonetheless.
In the following, I’ve overlayed the isotopes known in 1958 (red circles) over the isotopes inferred from the Ivy Mike hydrogen bomb explosion in 1952.
More isotopes have been found, red circles at top left. But that’s not the point that I want to make.
The point that I want to make is that the existence of isotopes inferred to exist in 1952, the open black squares at lower right, are not present in the 1958 table of isotopes. The reason is clear. Although these isotopes from 1952 were inferred to have been present, they were not directly detected and no attempt was made to measure the half lives of them.
So, event though the 1958 table has reliability ratings of A (perfect) to G (probably spurious), the isotopes inferred to exist in 1952 were given a reliability even below the G rating. Which in turn tells me that isotopes with even a G rating should not be immediately discarded.
Another inference I make is that the missing inferred isotopes look like fertile ground for searching for new isotopes. Not just those already inferred, but possibly up to the islands of stability by subjecting californium metal to massive bombardments of fast neutrons. This ios a totally different approach to the present trick of colliding two nuclei. Colliding two nuclei gives a result deficient in neutrons, and so has run the risk of bypassing the islands of stability.
mollwollfumble offers Lawrence’s suggesion (see thread E. O. Lawrence) of reaching critical mass and having a fast neutron chain reaction without initiating an explosion, as the best chance of finding these neutron-rich isotopes, and thus the islands of stability, if they exist.
You can think of the suggestion of Lawrence as being the nuclear equivalent of firing a bullet. A short term rapid pulse of energy that does not cause the gun to explode because it is not confined. As opposed to the slow release of energy in a nuclear reactor or in a fire.
Date: 24/07/2021 15:05:45
From: mollwollfumble
ID: 1769690
Subject: re: Who discovered the isotopes, and how?
Following on from the Ivy Mike (H-bomb) test, I came up with an idea of my own for finding new isotopes.
Part of the key to it is generating a very rapid nuclear chain reaction without a massive explosion.
It works on a principle similar to that used in making the first lasers, but instead of amplifying light or microwaves it amplifies neutrons.
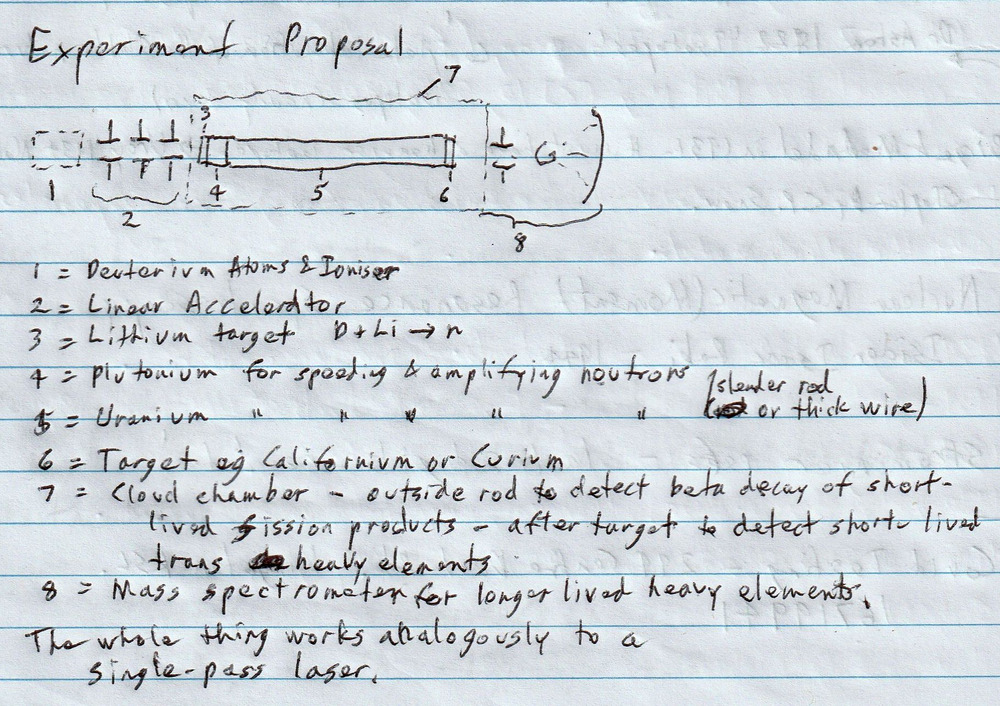
Starting with deuterium gas (1), this is ionised by a big pulse of energy and fed into a linear accelerator (2). At the output of the linear accelerator is a plug of lithium (3) that generates neutrons. The neutrons are amplified and accelerated through a rod of plutonium (4) and then through a thin rod or thick wire of natural or depleted uranium (5). At the end of the uranium is a target of either californium or curium (6), depending on how rich you are. Curium-244 is 185 thousand dollars a gram, Californium-252 is 60 million dollars a gram. Americium-243 is only 750 dollars a gram so is suitable for the first test run.
Surrounding the experiment is a cloud chamber (7) tuned to detect short period beta decays. This used to be routinely used in detecting half lives shorter than milliseconds, and is a lot cheaper than the more modern solid state detectors usin in the LHC for example.
Further down than the cloud chamber is a mass spectrometer (8) to collect and identify nuclides with half life timescales longer than milliseconds.
One thing that is necessary for this to work at all is the assumption that, as neutron energy increases, the neutrons departing a nuclear fission reaction become more aligned with those approaching. In justification of that, I refer you to Newton’s cradle (see below). A particle (fast neutron) hitting a collection of particles (nucleus) will tend to shed a single fast neutron heading off in the same direction. This is somewhat like the stimulated emission of radiation in a laser.
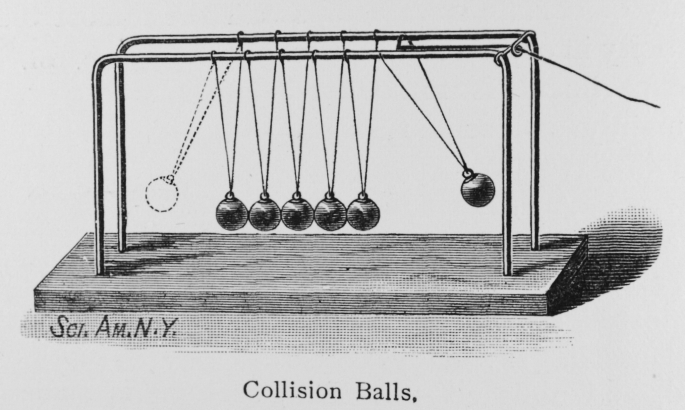
The other thing is that the cloud chamber adjacent to the uranium rod probably can’t work at the same time as that beyond the Curium or Californium target. Measurements in the cloud chamber beside the uranium rod will be dominated by fission products, which are exceedingly common. Beyond the target, I’m looking for exceedingly rare heavy nuclides. So the cloud chamber adjacent to the rod would be saturated with particles, masking results, long before the neutron flux is high enough to detect short-lived high mass nuclides from the target.
What’s so special about this proposal?
The unique combination of extremely high fast-neutron flux (fast enough for multiple neutron captures) and measurement of extremely short nuclide lifetimes, perhaps as small as 10^-11 seconds.
Date: 24/07/2021 20:17:33
From: mollwollfumble
ID: 1769906
Subject: re: Who discovered the isotopes, and how?
A piece of history here. Written in 1949 and declassified in 1955. It brings back memories of what science used to be like: manual typewriter, yellowed paper, hand-written equations, and scribbling in the margins. Nice to see that it’s made it onto the web.


Collecting radioactive products from a cyclotron in 1949. Note here this is protons rather than dueterium or alpha particles. Proton capture gives new elements with relatively few neutrons. In this case Carbon-10
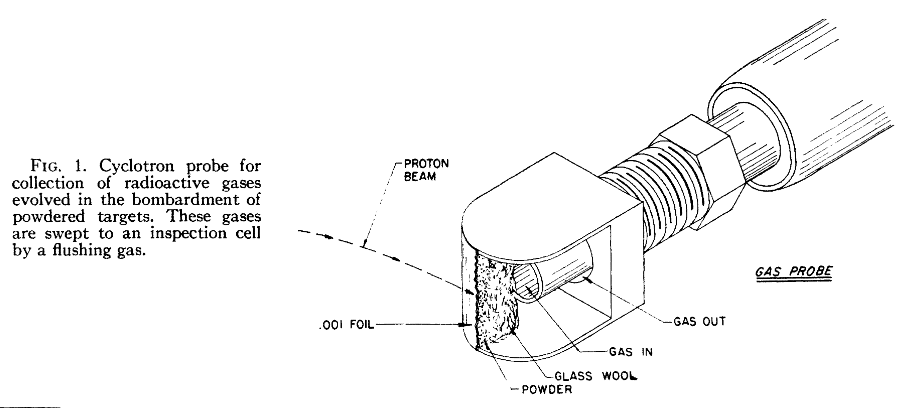
This uses the ‘gas flow and geiger counter’ method for determining half-lives. This method was also used for finding the isotope Helium-6 back in 1936, and seems to be a popular method for finding new isotopes of loght atoms.
The two other popular methods for measuring half-lives of new isotopes are Wilson cloud chanber, and mass spectrometry.
In the period 1936-1939, neutrons, deuterons and alpha particles were fired at all sorts of elements to find new isotopes. Isotopes including but not limited to I, Zn, Mn, Co, Fe, Sn etc
A bit later, circa 1946-1950, fission products were fed through “resin columns” to separate out each element, and then the result fed into a mass spectrometer to separate out each isotope. That’s a good method for long-lived isotopes.
Date: 25/07/2021 04:41:34
From: mollwollfumble
ID: 1769966
Subject: re: Who discovered the isotopes, and how?
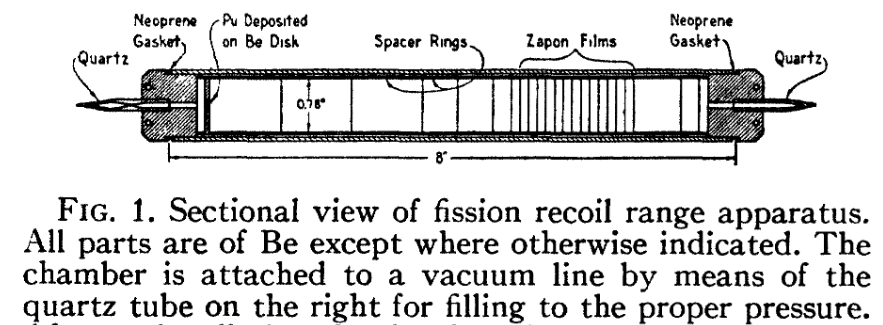
Just one more pre-1958.
This was an experiment to determine how far fission fragments from plutonium travelled in air.
Pre-1958 there are an uncountable number of researchers working on discovering and characterising new isotopes. From the summary paper from the chem department of the Uni of California (remember Californium) there are 68 pages of references, with about 64 references per page, and about 28 lead authors per page. That makes ~4,350 cited papers and ~1,900 cited lead authors. A quick check through the literature on Google Scholar and we could easily double that.
Taking some references from isotopes that interested me, I looked up lead authors Bjerge, Livingood, Hayden, Hahn, Sherr, Nelson and Katcoff. Most authors were in the USA, sometimes from places like Oak Ridge and Los Alamos. Not Hahn, who published in German.
I think it’s ironic that they call the place “Los Alamos” rather than “The Alamo”. Remember Los Alamos!
Bjerge used neutrons to irradiate Beryllium, and measured the half life using gas flow and geiger counter. The neutrons came from a radium-beryllium soiurce.
Livingood (great name) looked for isotopes of I, Zn, Mn, Co, Fe. Sn etc. firing neutrons , dueterons and alpha particles from the Berkeley cyclotron. A cloud chamber was used, as was “absorption in aluminium and lead”.
Hayden was a mass spectrogram expert. In addition to stable isotopes, he identified fission fragments that others had separated out using resin columns.
Hahn we know about. I opted not to translate from German.
Sherr looked into isotopes of carbon using the gas flow and geiger counter method.
Nelson & Pool from Oak Ridge looked into isotopes of Fe, Co, Ni, Cr, Mn using all four bombardment methods of alpha, neutron, gamma and deuteron.
Katcoff from Los Alamos was old-school in the sense of wet chemistry involving dissolving in HCl and quadruple distillation. Using gas sweeping and fractional precipitation etc. Stuidied itotopes of B, Tc, I, Ce, Pr, Y, La, Zr, Nb, Ar, Eu, Pd, Rh, Xe, etc.
————-
OK. that covers the discovery of isotopes up to 1958. What happened after that date?
Date: 26/07/2021 07:00:14
From: mollwollfumble
ID: 1770319
Subject: re: Who discovered the isotopes, and how?
> OK. that covers the discovery of isotopes up to 1958. What happened after that date?
I still don’t know. After 1958, tables of isotopes become difficult to obtain. Not on the web.
Lederer (1967) “Table of isotopes” is hard to come by.
For Browne (1978) “Table of isotopes”, I would need to apply to Oak Ridge directly
For Browne (1986) “Table of isotopes” is for sale for US$600 new or US$200 second hand.
so can’t use that.
1958 is also the first edition of the Nuclear Engineering Handbook
This little book review of Lederer (1967) includes a summary of how the number of known isotopes has increased with time. https://physicstoday.scitation.org/doi/abs/10.1063/1.3034044?journalCode=pto . The following are quotes from that book review.
47 radioactive isotopes in Fermi (1934)
71 in Fermi (1935)
230 radioactive and 290 stable nuclei in Livingood (1940)
~1150 nuclei in Seaborg (1948)
“By that time the literature on nuclei was forming an avalanch”
“New journal Nuclear Data”
In Lederer (1967) the bibliography contains 10,000 papers.
1824 nuclei in Lederer (1967)
“It is surprising how portable a tome of 594 pages containing probably $10 billion worth of data can be”.
—-
This one is interesting, from 1975 at Loa Alamos https://www.osti.gov/servlets/purl/4136569
England “ENDF/B-IV Fission-Product Files: Summary of Major Nuclide Data”
“Two and one-half years ago, a large task force was organized to expand the ENDF/B fission-product data from a 55-nuclide cross-section data set to a comprehensive file which, at present, encompasses data on 824 nuclides. Approximately 30 people from various industrial and government laboratories have cooperated in this task.”
“The motivation for an expanded file began with the need for a reference set of fission-product decay data for calculating decay heating during loss-of-coolant accidents”
“A complete listing of the decay and cross-section files requires several thousand pages. In this report … less than 30 pages. These tiles contain approximately 300 000 data entries.”

The lightest fission prodict is Cobalt-72, which decays through 5 steps to stable Germanium 72.
The heaviest fission product is Erbium-176, which is stable.
There’s no gap in the table between fragments of low energy and fragments of high energy. (In the earliest tables of fission fragments there was a gap).
——-
When it comes to fission products and reactor actinides and transuranics, computer codes are being developed beginning in the 1970s to predict what nuclides are present in nuclear reactors.and nuclear waste at any given time. https://www.osti.gov/servlets/purl/5352089
By Croff (1980), “ORIGIN2. is a versatile point depletion and decay computer code for use in simulating nuclear fuel cycles and calculating the nuclide compositions of materials contained therein. This code represents a revision and update of the original ORIGIN computer code which has been distributed world-wide beginning in the early 1970s.”
“ORIGIN would form the basis for the study and design of fuel reprocessing plants, spent fuel shipping casks, waste treatment and disposal facilities, and waste shipping casks” using the Table of Isotopes by Lederer (1967). ORIGIN was not sufficient for envirnmental impact assessments or for accurate prediction of the state of the reactor core. ORIGIN2 was designed to comprehensive enough to cope with these as well.
——-
Date: 26/07/2021 07:49:37
From: mollwollfumble
ID: 1770321
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
> OK. that covers the discovery of isotopes up to 1958. What happened after that date?
I still don’t know. After 1958, tables of isotopes become difficult to obtain. Not on the web.
Lederer (1967) “Table of isotopes” is hard to come by.
For Browne (1978) “Table of isotopes”, I would need to apply to Oak Ridge directly
For Browne (1986) “Table of isotopes” is for sale for US$600 new or US$200 second hand.
so can’t use that.
1958 is also the first edition of the Nuclear Engineering Handbook
This little book review of Lederer (1967) includes a summary of how the number of known isotopes has increased with time. https://physicstoday.scitation.org/doi/abs/10.1063/1.3034044?journalCode=pto . The following are quotes from that book review.
47 radioactive isotopes in Fermi (1934)
71 in Fermi (1935)
230 radioactive and 290 stable nuclei in Livingood (1940)
~1150 nuclei in Seaborg (1948)
“By that time the literature on nuclei was forming an avalanch”
“New journal Nuclear Data”
In Lederer (1967) the bibliography contains 10,000 papers.
1824 nuclei in Lederer (1967)
“It is surprising how portable a tome of 594 pages containing probably $10 billion worth of data can be”.
—-
This one is interesting, from 1975 at Loa Alamos https://www.osti.gov/servlets/purl/4136569
England “ENDF/B-IV Fission-Product Files: Summary of Major Nuclide Data”
“Two and one-half years ago, a large task force was organized to expand the ENDF/B fission-product data from a 55-nuclide cross-section data set to a comprehensive file which, at present, encompasses data on 824 nuclides. Approximately 30 people from various industrial and government laboratories have cooperated in this task.”
“The motivation for an expanded file began with the need for a reference set of fission-product decay data for calculating decay heating during loss-of-coolant accidents”
“A complete listing of the decay and cross-section files requires several thousand pages. In this report … less than 30 pages. These tiles contain approximately 300 000 data entries.”

The lightest fission prodict is Cobalt-72, which decays through 5 steps to stable Germanium 72.
The heaviest fission product is Erbium-176, which is stable.
There’s no gap in the table between fragments of low energy and fragments of high energy. (In the earliest tables of fission fragments there was a gap).
——-
When it comes to fission products and reactor actinides and transuranics, computer codes are being developed beginning in the 1970s to predict what nuclides are present in nuclear reactors.and nuclear waste at any given time. https://www.osti.gov/servlets/purl/5352089
By Croff (1980), “ORIGIN2. is a versatile point depletion and decay computer code for use in simulating nuclear fuel cycles and calculating the nuclide compositions of materials contained therein. This code represents a revision and update of the original ORIGIN computer code which has been distributed world-wide beginning in the early 1970s.”
“ORIGIN would form the basis for the study and design of fuel reprocessing plants, spent fuel shipping casks, waste treatment and disposal facilities, and waste shipping casks” using the Table of Isotopes by Lederer (1967). ORIGIN was not sufficient for envirnmental impact assessments or for accurate prediction of the state of the reactor core. ORIGIN2 was designed to comprehensive enough to cope with these as well.
——-
> “New journal Nuclear Data”
The Journal is actually called “Nuclear Data Sheets”, There are 172 volumes by 2021, 52 volumes by 1987, 18 volumes by 1976, 4 volumes by 1968. Articles tend to concentrate on a specific atomic mass. For example in 2021, one article reviews data for atomic mass 252, which covers elements Uranium to Lawrencium.
By 1987, there is so much nuclear data that there are organisations all around the world dedicated to summarising nuclear data. Each of these organisations is tasked with maintaining a database for a specific nuclear mass range. eg. Japan’s role is to keep up to date with published nuclides in the atomic mass range of 118 to 129. These organisations were:

Date: 26/07/2021 23:37:02
From: mollwollfumble
ID: 1770649
Subject: re: Who discovered the isotopes, and how?
Moving forward from 1958 to 1971.
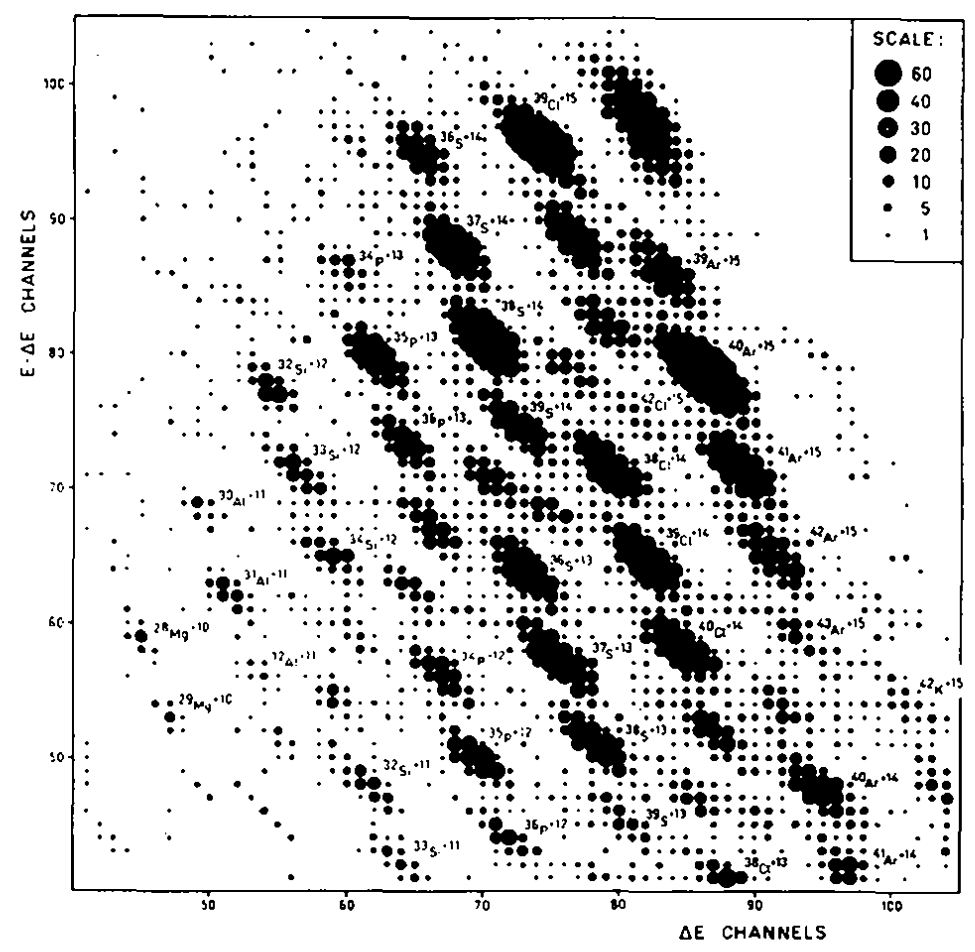
Artukh announced the discovery of 17 new isotopes in a single paper.
https://hal.archives-ouvertes.fr/file/index/docid/214836/filename/ajp-jphyscol197132C618.pdf
And here they are, shown below, as detected by the mass spectropmeter. A bit difficult to see, but each dot is an isotope of Mg, Al, Si, P, S or Cl (new isotopes) as well as Ar, K (known isotopes).
Several parts of the method used here are distinctly new, approaching modern. Gone are the dueterium bullets, instead they use the 40-Ar nucleus as bullets. Gone are the cloud chamber, the geiger counter and the collecting plates from earlier types of experiment. Instead we have paired silicon detectors (similar to those on the CERN LHC detectors) at the business end of their mass spectrometer. (For some unknown reason they refer to the mass spectrometer as a “telescope”, I have no idea why).
New to me is how they use the paired silicon detectors. One is thin and measures the Energy E of the mass fragments. The other is thin and measures the change in energy deltaE as the mass fragment is slowed by the silicon. The magnetic field separates the isotopes one way, in space, and the map of E vs DeltaE separates isotopes another way.
The use of a mass spectrometer means that isotopes can be detected with quite short half lives, perhaps down to 10^-8 seconds.
The 40-Ar nuclei are spun up to energy in the Dubna 310 cm heavy ion cyclotron in Moscow. (Remember element Dubnium) The researchers are French.
A historical note from the paper is that before 40-Ar was used, some success had already been had using 15-N, 18-O and 22-Ne as nuclear bullets. I have heard of 48-Ca being used in more recent studies.
Date: 27/07/2021 09:51:26
From: mollwollfumble
ID: 1770692
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
Moving forward from 1958 to 1971.
Artukh announced the discovery of 17 new isotopes in a single paper.
https://hal.archives-ouvertes.fr/file/index/docid/214836/filename/ajp-jphyscol197132C618.pdf
And here they are, shown below, as detected by the mass spectropmeter. A bit difficult to see, but each dot is an isotope of Mg, Al, Si, P, S or Cl (new isotopes) as well as Ar, K (known isotopes).
Several parts of the method used here are distinctly new, approaching modern. Gone are the dueterium bullets, instead they use the 40-Ar nucleus as bullets. Gone are the cloud chamber, the geiger counter and the collecting plates from earlier types of experiment. Instead we have paired silicon detectors (similar to those on the CERN LHC detectors) at the business end of their mass spectrometer. (For some unknown reason they refer to the mass spectrometer as a “telescope”, I have no idea why).
New to me is how they use the paired silicon detectors. One is thin and measures the Energy E of the mass fragments. The other is thin and measures the change in energy deltaE as the mass fragment is slowed by the silicon. The magnetic field separates the isotopes one way, in space, and the map of E vs DeltaE separates isotopes another way.
The use of a mass spectrometer means that isotopes can be detected with quite short half lives, perhaps down to 10^-8 seconds.
The 40-Ar nuclei are spun up to energy in the Dubna 310 cm heavy ion cyclotron in Moscow. (Remember element Dubnium) The researchers are French.
A historical note from the paper is that before 40-Ar was used, some success had already been had using 15-N, 18-O and 22-Ne as nuclear bullets. I have heard of 48-Ca being used in more recent studies.

Moving on from 1971 to 1978. This paper is from Darmstadt in Germany (remember element Darmstadtium). Z. Physik A 288, 189- 192 (1978).
Schrewe, “Evidence of New Isotopes: 169,170 lr 166,167,168 Re”
https://sci-hub.st/https://link.springer.com/article/10.1007%2FBF01408649
“The linear accelerator UNILAC (Universal Linear Accelerator) is the starting point of acceleration of ions. On a length of 120 meters ions of all kinds can …”
“Until recently, scarce information on very neutron deficient Ir isotopes was available. The light Ir isotopes down to mass number 171 have been produced by bombardment of enriched Er isotopes using F-19, and of Tm using O-16 ions, respectively.”
“Reaction recoils emitted from the targets were entered into a gas-filled chamber, stopped and transported with argon as well as helium through 6 m of capillary. A target set-up inside the stopping chamber. Collection was done onto a wheel, the wheel rotated to bring the activity in front of the (silicon) detectors.”
This is largely old school, a long capilllary will lose all short-lived isotopes between irradiation and detection. What is new-school is the purification of the purification of the Krypton isotopes used in the bombardment and the tuning of bombardment energy to maximise the yield of specific product isotopes.
——
Being neutron-deficient isotopes, these decay by alpha particle radiation, and the radiation energy and half life was measured.
Moving from 1978 to 1985. Same machine, the UNILAC at Darmstadt (element darmstadtium again) but this time with a much improved detector.
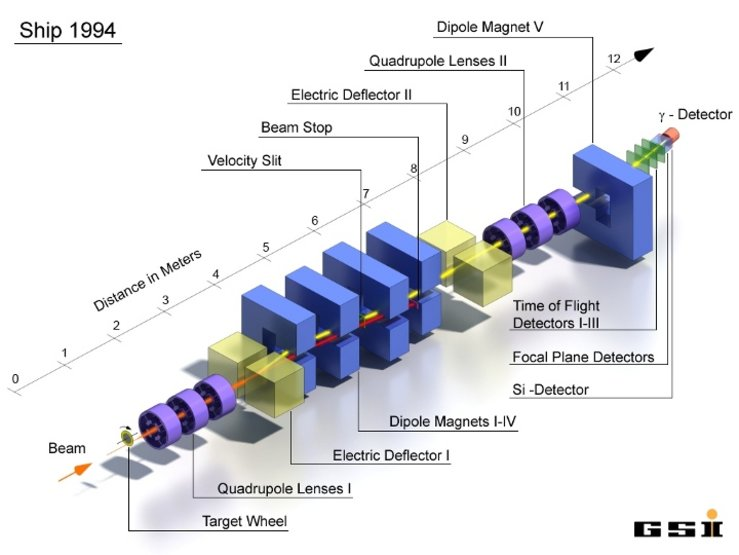
Article by Hessberger finding four more isotopes of Lawrencium and element 105. https://sci-hub.st/https://link.springer.com/article/10.1007/BF01415134
This work follows on from from the discovery of elements 107, 108 and 109 by Ogranessian in Moscow and others a few years earlier.
Hessberger (1985) fired Ti-50 at 209-Bi. Why Ti-50? Because if is close to the doubly magic high neutron nucleus of Ca-48, and the fusion interaction usually ends up shedding a neutron or two. This is known as a “cold fusion” reaction because it is done at an energy below the Coulomb barrier for the target and projectile; if it was done close to the coulomb barrier then the result would by a fission recion insread of a capture.
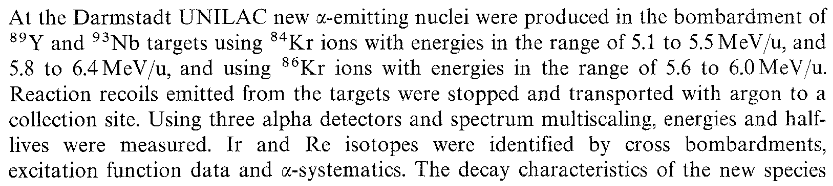
The new detector used is known as SHIP (it’s good fun to read about the 1994 version in https://www.gsi.de/work/forschung/nustarenna/nustarenna_divisions/she_physik/experimental_setup/ship). SHIP separates the new isotopes from the particle beam using a “velocity filter”. Here’s an image of SHIP. The new “Time of Flight” detector is the key component in 1985.
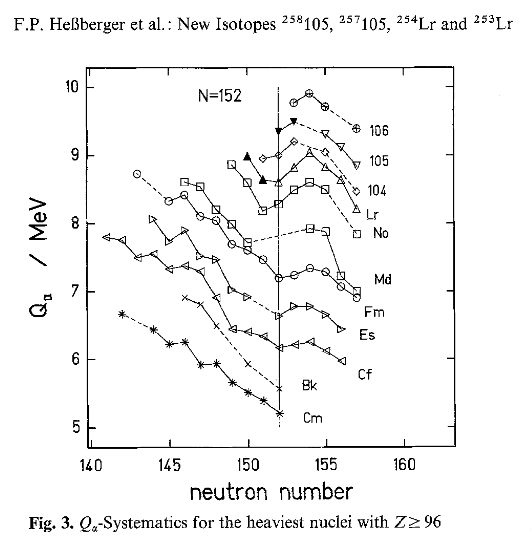
Results from Hessberger (1985). Here Qa is the elpha decay energy.
Date: 27/07/2021 12:54:51
From: mollwollfumble
ID: 1770738
Subject: re: Who discovered the isotopes, and how?
Enough of Germany, Russia and the USA for a while.
Let’s move on from 1985 in Germany to 1990 in Japan.
https://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/77333/1/chd068_2_139.pdf
Okano (1990) “Identification of New Isotopes with the He-Jet Fed On-Line Isotope Separator KUR-ISOL”
The target material of 93% enriched 235U is irradiated by neutrons.There are four new isotopes found among fission fragments: 152Ce, 154Pr 155Nd and 156Pm. This is an old-style projectile and an old style gas flow isotope separator, Helium is used as the gas. The fission fragments are slowed down to thermal equilibrium by the He. The mass separated radioisotopes are collected on an Al-coated Mylar tape, again not a particularly new method. So it’s pretty amazing that new isotopes have been found this recently.
An alternative to aerosol collection is oxidisation. When ionisation does not suffice.
New isotopes have half lives in the range of 3 to 30 seconds. Data is analysed using a Fortran computer program.
There are improvements in theory, as shown below. From “gross theory” to “microscopic theory”, applied to Cerium isotopes.
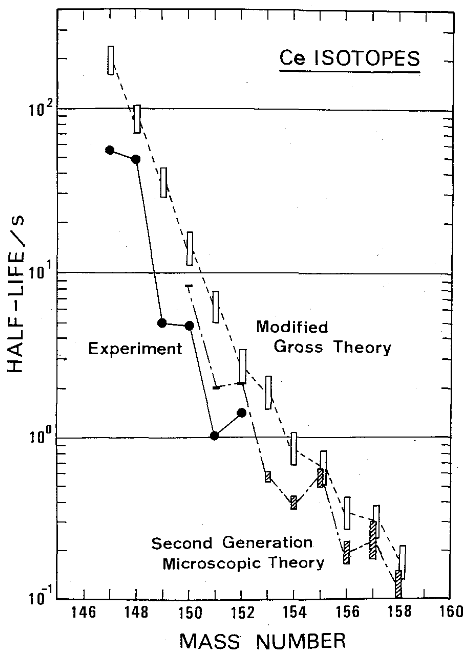
Nuclear deformation comes from moment of inertia calculations. I don’t fully understand how this works. It seems to imply that heavy nuclei are protate and spinning with axis along their long dimension, which is the opposite of what I’ve expect from the balance between the neclear binding force and the centrifugal force.

Date: 27/07/2021 16:14:31
From: mollwollfumble
ID: 1770791
Subject: re: Who discovered the isotopes, and how?
Moving on from 1995 and Japan, to 1999 and back to Darmstadt.
http://fpsalmon.usc.es/genp/doc/mecanismos/bibliografia/article19.pdf
By this time there are getting to be more authors per publication. In this case auithors are from Warsaw, Darmstadt, France, Guildford and Oak Ridge. “Seven previously unobserved neutron-rich isotopes 209Hg, 210Hg, 211Tl, 212Tl, 218Bi, 219Po and 220Po have been identified among the fragmentation products of a 1000 MeV/nucleon 238U beam incident on a beryllium target. The gamma-ray decays of 9 known and 4 new microsecond-isomers in nuclei around 208 Pb were also observed.”
Why fire U238 at Be rather than the other way around? LOL.
With this facility, isotopes with half-lives in the range of 10 nanoseconds to 1 millisecond can be observed.
This work was done on the SIS machine, the heavy ion synchoton. I’d been wondering how long it would take for synchrotrons to take a part in finding new isotopes. The previous ones I reviewed above were all either fixed decay sources such as radium, cyclotrons or linear accelerators such as UNILAC. Synchrotrons can give a higher energy than all of those three.
From wikipedia. “The UNILAC was commissioned in 1975; the SIS 18 and the ESR were added in 1990 boosting the ion acceleration from 10% of light speed to 90%.” The FRS, also used in the present paper, is the fragment separator. ESR, the experimental storage ring, is not used in the paper; it is used for accurate measurements of decay energies of new radioactive ions trapped in the ESR ring.
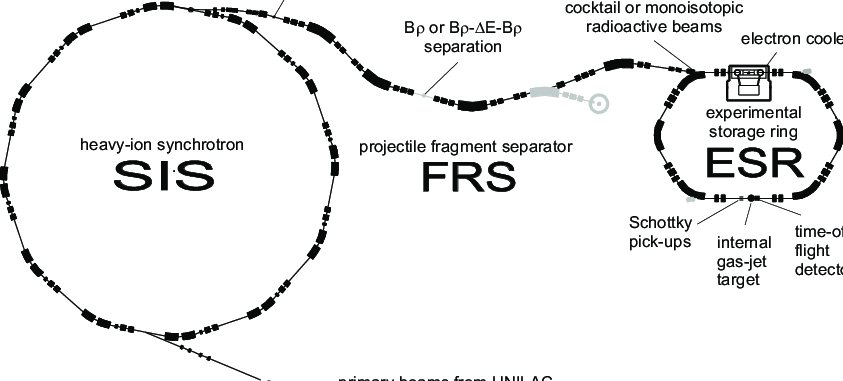
“To achieve the maximal efficiency for electron stripping, thin niobium foils of thickness 260 mm and 130 mm, respectively, were mounted downstream from the target and degrader. The resultant probability for fully stripped ions was measured to be 93% in the first stage and more than 75% in the second stage of the FRS.”
“The atomic number Z and mass-to-charge ratio ArQ of reaction product ions reaching the final focus were determined by combining measurements of time of flight TOF. with information from position-sensitive multi-wire counters and the magnetic field values.”
In the following figure, the framed numbers represent new isotopes.
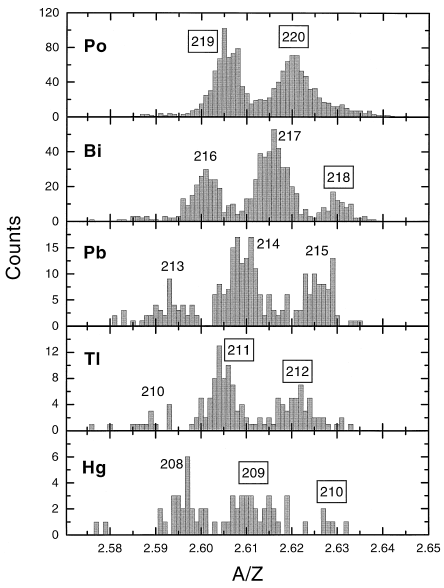
Date: 27/07/2021 17:40:58
From: mollwollfumble
ID: 1770804
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
Moving on from 1995 and Japan, to 1999 and back to Darmstadt.
http://fpsalmon.usc.es/genp/doc/mecanismos/bibliografia/article19.pdf
…

…
In the following figure, the framed numbers represent new isotopes.

> Moving on from 1995 and Japan, to 1999 and back to Darmstadt.
Oops, I meant from 1990 in Japan.
Let’s go back to Japan for the year 2010. Up to about 80 authors for this paper. https://journals.jps.jp/doi/pdf/10.1143/JPSJ.79.073201
“Identification of 45 New Neutron-Rich Isotopes Produced by In-Flight Fission of a 238U Beam at 345 MeV/nucleon”
(Note, the 1999 paper was a 238U Beam at 1000 MeV/nucleon, so this paper from 2010 is slower).
“RI Beam Factory at the RIKEN Nishina Center. Fission fragments were analyzed and identified by using the superconducting in-flight separator BigRIPS. We observed 45 new neutron-rich isotopes: 71Mn,73;74Fe, 76Co, 79Ni, 81;82Cu, 84;85Zn, 87Ga, 90Ge, 95Se, 98Br, 101Kr, 103Rb, 106;107Sr, 108;109Y, 111;112Zr,114;115Nb, 115;116;117Mo, 119;120Tc, 121;122;123;124Ru, 123;124;125;126Rh, 127;128Pd, 133Cd, 138Sn, 140Sb, 143Te,145I, 148Xe, and 152Ba.”
That’s an enormous number of new isotopes.
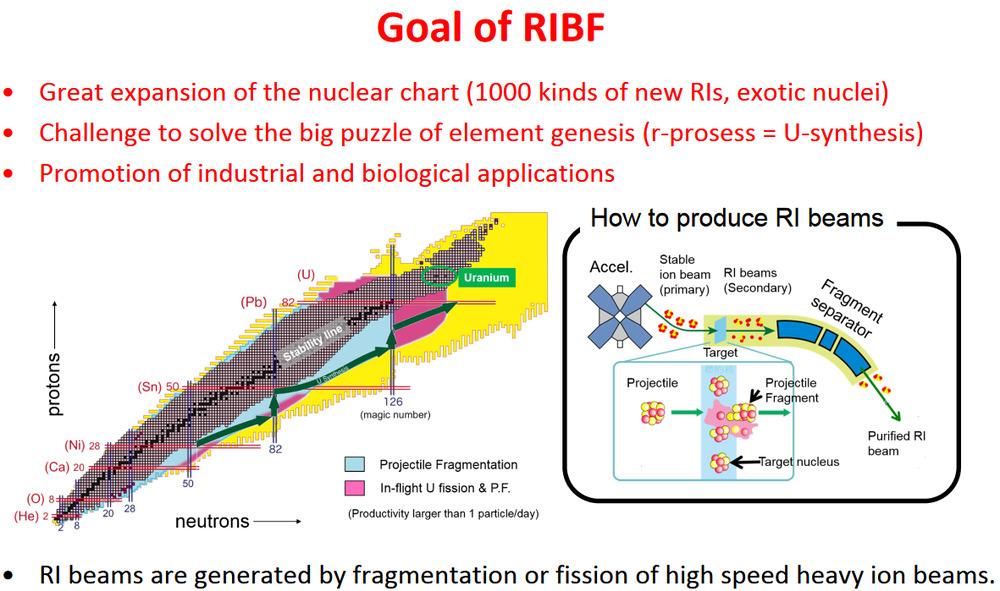
The’s have a look at the RIKEN Nishina Center. This slideshow is well worth a look through. https://accelconf.web.cern.ch/cyclotrons2016/talks/moa01_talk.pdf The source of high energy 238U atoms is called SRC, for Superconducting Ring Cyclotron, or for source ;-)

(By RI they mean radioisotope). The process by which all the elements on Earth were generated is approximately known from theiry, but this equipment will find out by actual experiment.
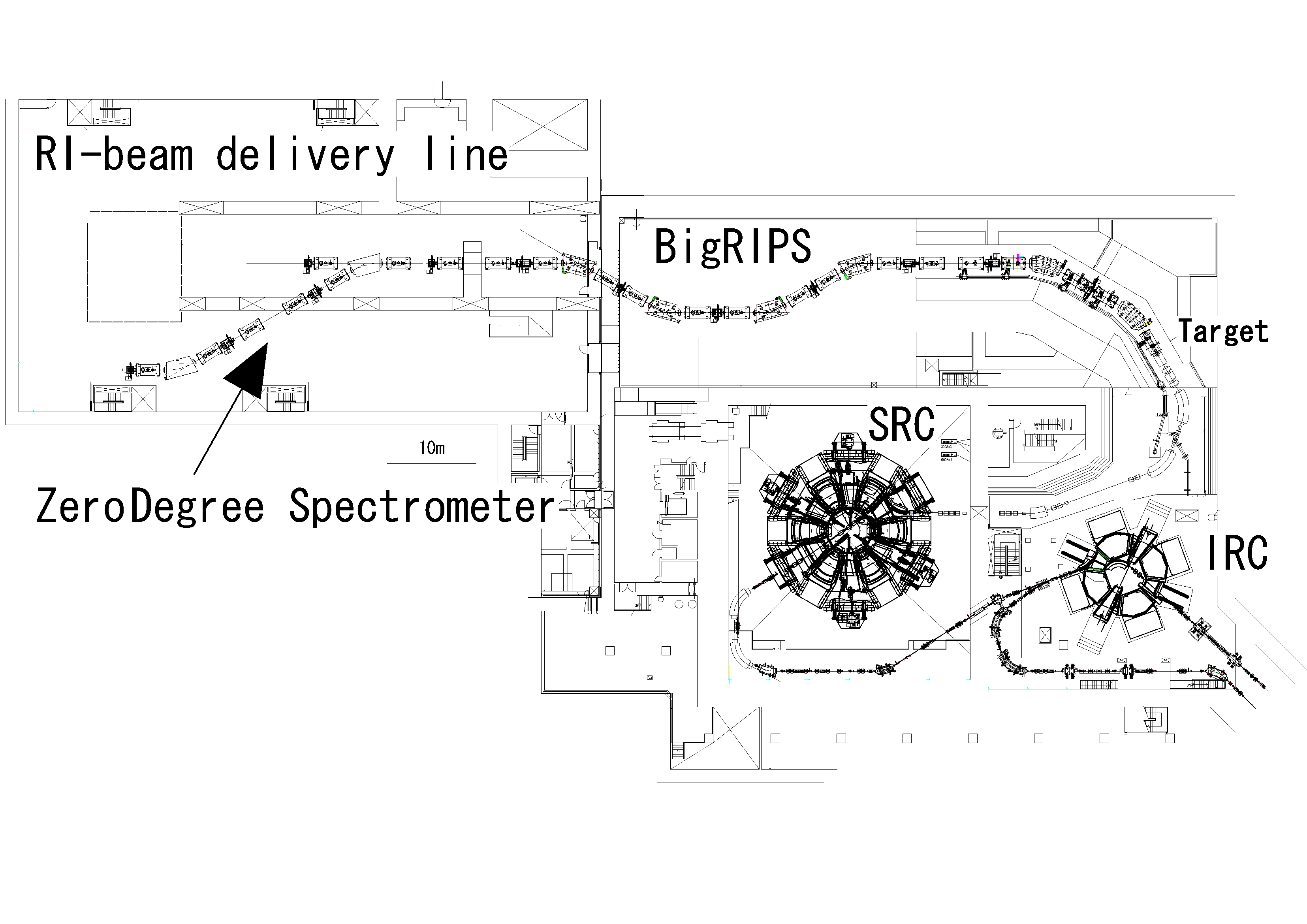
“The BigRIPS separator has been designed to be a two-stage RI beam separator. The first stage from the production target to the F2 focus comprises a two-bend achromatic spectrometer. This first stage serves to produce and separate RI beams. The second stage of BigRIPS separator is employed to identify RI-beam species. Position-sensitive detectors, timing detectors and δ E detectors are placed at the focuses of the second stage to measure the Bρ (magnetic field by density) value, the time-of-flight and the energy loss of RI beams. The scheme allows one to determine the atomic number (Z), the ratio of mass number to charge number (A/q) and the momentum (P) in an event-by-event mode.The total length is 77 m.”
Now back to the paper from 2010.
A 238U beam was fired at two targets in different experiments. “Beryllium because the so-called abrasion-fission (AF) process is more favorable for the production of isotopes in the Z ~ 30 to ~40 regions” and “a lead target, because the Coulomb-fission (CF) process that leads to asymmetric fission is more favorable for the production of isotopes in the Z ~ 50 region.”
The time of flight “TOF was measured between two thin plastic scintillation counters at focal points”. “The delta E was measured using a multi-sampling ionization chamber (MUSIC)”. “trajectory reconstruction of fragments were measured by using two sets of position-sensitive parallel plate avalanche counters”.
In the following plot, each smudged dot is a detected isotope. The lower figures are just zooming in on the upper figures.
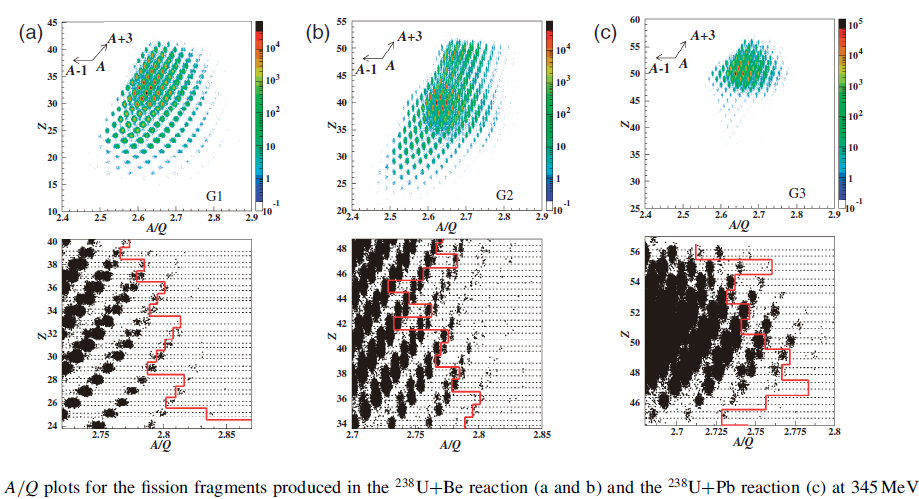
The new isotopes placed on the chart of nuclides. These are all neutron rich. Unusually for an article about finding new isotopes, half lives were not mentioned.
Date: 27/07/2021 17:59:16
From: Bubblecar
ID: 1770811
Subject: re: Who discovered the isotopes, and how?
Well done but they ought to be wearing hard hats.
Date: 27/07/2021 18:13:06
From: mollwollfumble
ID: 1770815
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
Let’s go back to Japan for the year 2010. Up to about 80 authors for this paper. https://journals.jps.jp/doi/pdf/10.1143/JPSJ.79.073201
“Identification of 45 New Neutron-Rich Isotopes Produced by In-Flight Fission of a 238U Beam at 345 MeV/nucleon”
(Note, the 1999 paper was a 238U Beam at 1000 MeV/nucleon, so this paper from 2010 is slower).
“RI Beam Factory at the RIKEN Nishina Center. Fission fragments were analyzed and identified by using the superconducting in-flight separator BigRIPS. We observed 45 new neutron-rich isotopes: 71Mn,73;74Fe, 76Co, 79Ni, 81;82Cu, 84;85Zn, 87Ga, 90Ge, 95Se, 98Br, 101Kr, 103Rb, 106;107Sr, 108;109Y, 111;112Zr,114;115Nb, 115;116;117Mo, 119;120Tc, 121;122;123;124Ru, 123;124;125;126Rh, 127;128Pd, 133Cd, 138Sn, 140Sb, 143Te,145I, 148Xe, and 152Ba.”
That’s an enormous number of new isotopes.
The’s have a look at the RIKEN Nishina Center. This slideshow is well worth a look through. https://accelconf.web.cern.ch/cyclotrons2016/talks/moa01_talk.pdf The source of high energy 238U atoms is called SRC, for Superconducting Ring Cyclotron, or for source ;-)

(By RI they mean radioisotope). The process by which all the elements on Earth were generated is approximately known from theiry, but this equipment will find out by actual experiment.

“The BigRIPS separator has been designed to be a two-stage RI beam separator. The first stage from the production target to the F2 focus comprises a two-bend achromatic spectrometer. This first stage serves to produce and separate RI beams. The second stage of BigRIPS separator is employed to identify RI-beam species. Position-sensitive detectors, timing detectors and δ E detectors are placed at the focuses of the second stage to measure the Bρ (magnetic field by density) value, the time-of-flight and the energy loss of RI beams. The scheme allows one to determine the atomic number (Z), the ratio of mass number to charge number (A/q) and the momentum (P) in an event-by-event mode.The total length is 77 m.”

Now back to the paper from 2010.
A 238U beam was fired at two targets in different experiments. “Beryllium because the so-called abrasion-fission (AF) process is more favorable for the production of isotopes in the Z ~ 30 to ~40 regions” and “a lead target, because the Coulomb-fission (CF) process that leads to asymmetric fission is more favorable for the production of isotopes in the Z ~ 50 region.”
The time of flight “TOF was measured between two thin plastic scintillation counters at focal points”. “The delta E was measured using a multi-sampling ionization chamber (MUSIC)”. “trajectory reconstruction of fragments were measured by using two sets of position-sensitive parallel plate avalanche counters”.
In the following plot, each smudged dot is a detected isotope. The lower figures are just zooming in on the upper figures.

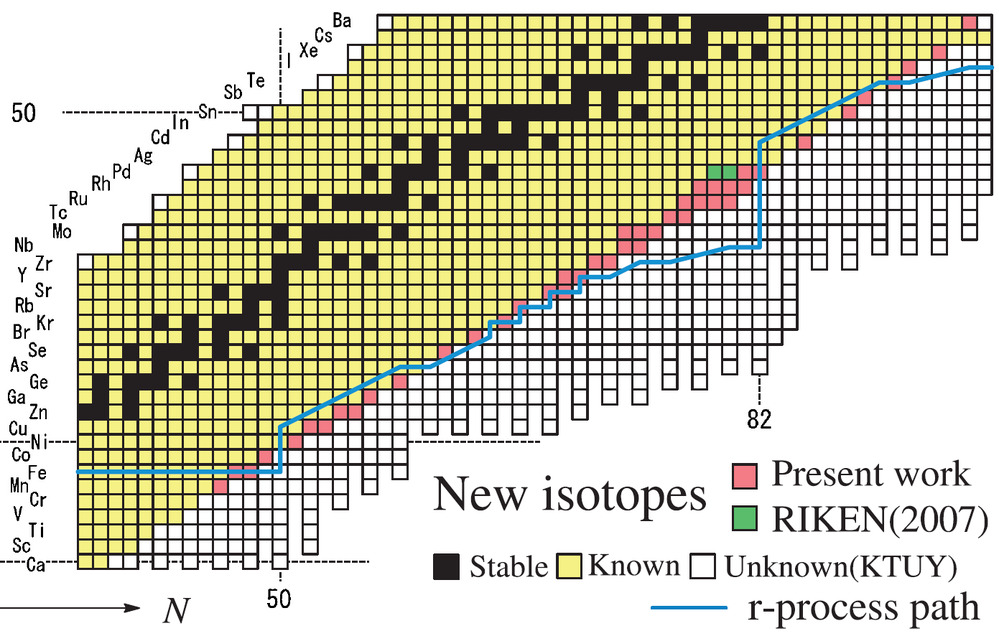
The new isotopes placed on the chart of nuclides. These are all neutron rich. Unusually for an article about finding new isotopes, half lives were not mentioned.

I just want to draw your attention to the chart below. This is the top of the table of nuclides from Karlsruhe (2018).
The line of maximum stability is the the top edge of the grey region, the grey region representing decay by beta radiation.
The top edge of the grey region stops at element 99, Einsteinium.
Every element heavier than Einsteinium is only known from neutron-poor isotopes that are off the line of maximum stability.
The postulated “islands of stability” for superheavy elements. One postulated “island of stability” is supposed to occur for Element 114. So, whereas we have now measured up to element 118, we’ve passed nowhere near the point of maximum stability for Element 114, so the postulated “islands of stability” may still exist.
Date: 27/07/2021 23:02:17
From: mollwollfumble
ID: 1770875
Subject: re: Who discovered the isotopes, and how?
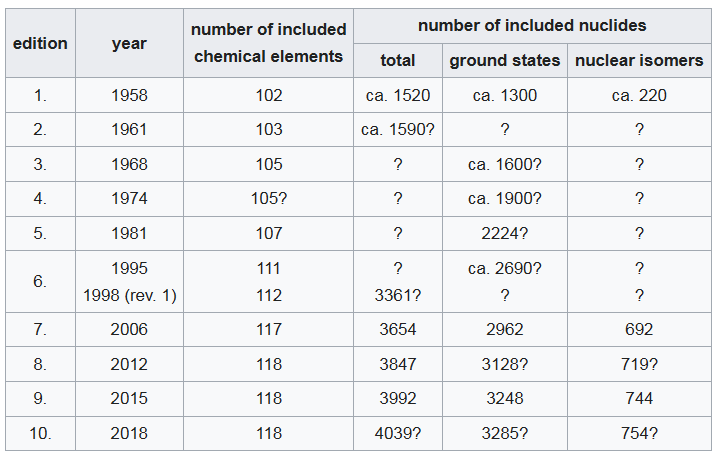
Wikipedia has a rough estimate of how many isotopes have been discovered in total, by year.
We’re up to 4,000 now, not just circa 3,000.
Date: 28/07/2021 02:58:20
From: mollwollfumble
ID: 1770902
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
Wikipedia has a rough estimate of how many isotopes have been discovered in total, by year.
We’re up to 4,000 now, not just circa 3,000.

The Russian centre for isotope discovery. It looks like an ordinary 2-storey Russian building from the outside, nothing to distinguish it as unusual. Perhaps because it was secretly opened in 1956 by Nikita Khrushchev. I think it’s at Dubna. The superheavy element factory SHEF is brand new and may or may not be open yet. Construction of SHEF began in 2017, and by 2019 there was an announcement that it would be open “by spring”, but nothing since.
This Flerov facility is famous for superheavy element Flerovium (number 114). Yuri Oganessian (element 118) works there.
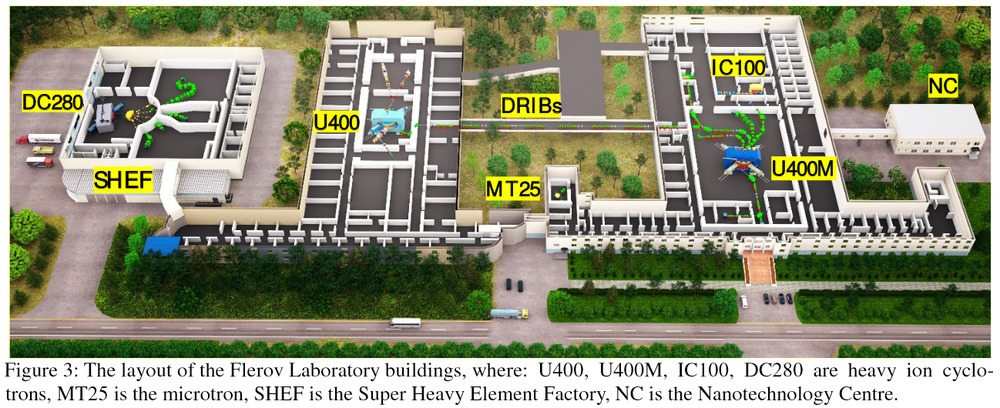
Date: 28/07/2021 09:00:29
From: mollwollfumble
ID: 1770921
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
The Russian centre for isotope discovery. It looks like an ordinary 2-storey Russian building from the outside, nothing to distinguish it as unusual. Perhaps because it was secretly opened in 1956 by Nikita Khrushchev. I think it’s at Dubna. The superheavy element factory SHEF is brand new and may or may not be open yet. Construction of SHEF began in 2017, and by 2019 there was an announcement that it would be open “by spring”, but nothing since.
This Flerov facility is famous for superheavy element Flerovium (number 114). Yuri Oganessian (element 118) works there.

The superheavy element factory did open, “On 25 March 2019”. See http://www.jinr.ru/posts/inauguration-of-the-factory-of-superheavy-elements/ Its core is the DC-280 cyclotron. See also https://link.springer.com/article/10.1134/S1547477119060177 .
“The main task of the new accelerator is to implement a long-term research program at the SHE Factory aimed at the synthesis of new elements (Z ≥ 119) and a detailed study of nuclear–physical and chemical properties of previously discovered elements nos. 112–118.”
For a timelapse video of its construction that is fun to watch, see https://youtu.be/jjJjGVN36Hg . The wedge-shaped elements are superconducting magnets.
An earlier article about the device says:
Instead of 48Ca, the SHE is starting using 84Kr ions.
Date: 28/07/2021 09:57:11
From: mollwollfumble
ID: 1770943
Subject: re: Who discovered the isotopes, and how?
Isotope discovery in the future, beyond 2026.
I saw this coming – but only after it had already occurred !
One way to make neutron-rich nuclides is to bombard existing nuclides with electrons. Reying on the reaction proton+electron=neutron. This is the same as inverting neutron decay, which is the same as inverting beta decay.
Already this has been done starting with Californium-252, bombarding it with electrons to make brand new isotopes Am-252, Pu-252, Np-252. I don’t know where this was done.
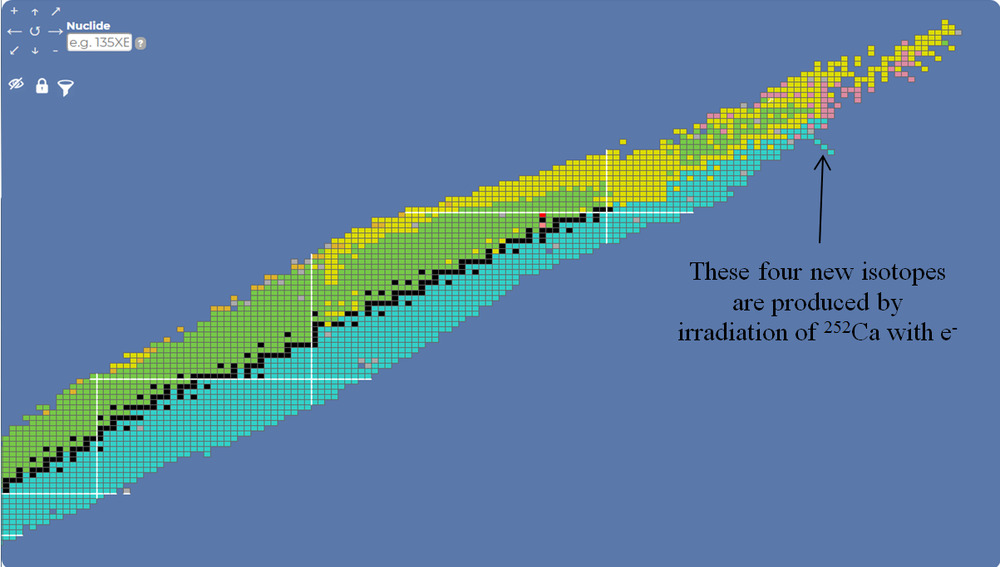
Ideally we want to do this with isotopes heavier than Ca-252 because with Ca-252 the process is moving away from the line of maximum stability. With heavier isotopes we’d be moving towards the line of maximum stability, but these heavier isotopes are radioactive with short half lives, so what to do?
Now we have a proposal just this year, 2021. http://publications.jinr.ru/record/153960/files/Grigorenko2021_Article_DERICAProjectAndStrategiesOfTh.pdf DERICA stands for the Dubna Electron–Rare Isotope Collider Facility. Newly made isotopes are transferred from a synchrotron into a storage ring called the e-ring where the are bombarded by electrons from a linear accelerator.
“The project is partitioned into relatively short scientifically motivated stages. The first stages of the project will be able to furnish scientific results in about three to five years after the beginning of the construction.”
Date: 28/07/2021 10:11:18
From: mollwollfumble
ID: 1770946
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
Isotope discovery in the future, beyond 2026.
I saw this coming – but only after it had already occurred !
One way to make neutron-rich nuclides is to bombard existing nuclides with electrons. Reying on the reaction proton+electron=neutron. This is the same as inverting neutron decay, which is the same as inverting beta decay.
Already this has been done starting with Californium-252, bombarding it with electrons to make brand new isotopes Am-252, Pu-252, Np-252. I don’t know where this was done.

Ideally we want to do this with isotopes heavier than Ca-252 because with Ca-252 the process is moving away from the line of maximum stability. With heavier isotopes we’d be moving towards the line of maximum stability, but these heavier isotopes are radioactive with short half lives, so what to do?
Now we have a proposal just this year, 2021. http://publications.jinr.ru/record/153960/files/Grigorenko2021_Article_DERICAProjectAndStrategiesOfTh.pdf DERICA stands for the Dubna Electron–Rare Isotope Collider Facility. Newly made isotopes are transferred from a synchrotron into a storage ring called the e-ring where the are bombarded by electrons from a linear accelerator.
“The project is partitioned into relatively short scientifically motivated stages. The first stages of the project will be able to furnish scientific results in about three to five years after the beginning of the construction.”
errata -
You know I meant 252Cf not 252Ca, right.
And the fourth new isotope is 252Cm with a half life of two days (which is quite long!)
Let’s try again
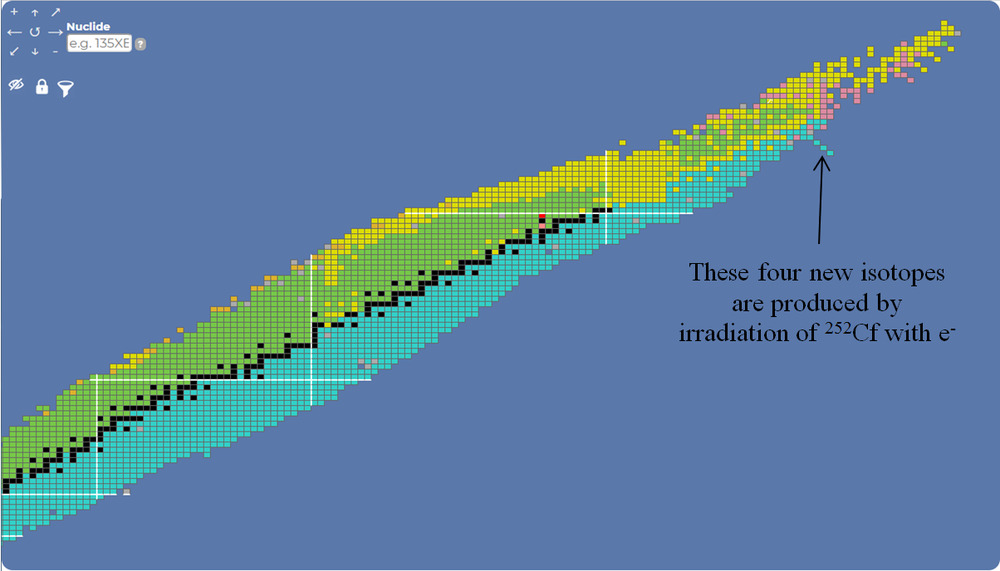
Date: 28/07/2021 17:48:04
From: mollwollfumble
ID: 1771105
Subject: re: Who discovered the isotopes, and how?
Most modern isotope discoveries have come from Germany (Darmstadt), Japan (RIKEN), and Russia (Dubna). But I happened to note that a couple of new isotopes have come from China.
China has for a decade had some good computer software for predicting the best way to discover new superheavy nuclei, but has not followeed that up on the experimental side.
So I was delighted to find “New heavy isotopes 259Db and 265Bh were also synthesized at HIRFL in Lanzhou (China)”
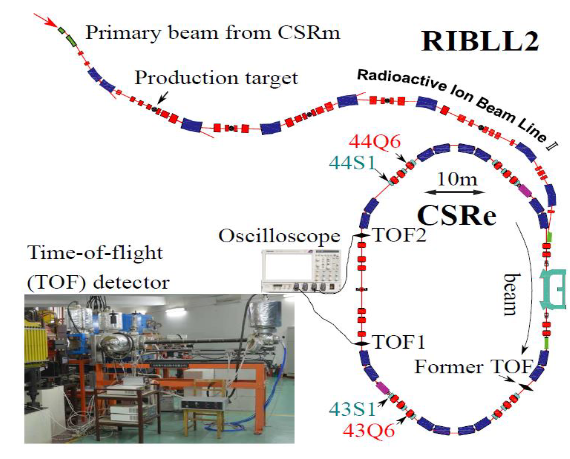
https://iopscience.iop.org/article/10.1088/1742-6596/1401/1/012003/pdf is titled “Present status of HIRFL complex in Lanzhou”.
“HIRFL is one of the largest heavy ion research facility in China. It belongs to the National Laboratory of Heavy Ion Accelerator, which was established in 1991, at Institute of Modern Physics (IMP). HIRFL consists of two cyclotrons (SFC and SSC), one synchrotron (CSRm) and one storage ring spectrometer (CSRe), in chain. The SFC cyclotron was constructed in 1960s for light ions. It’s upgraded in 1980s to accelerate heavy ions from hydrogen to uranium, as required to be an injector of cyclotron SSC.”

Heavy ion beams available at HIRFL
The product storage ring CSRe contains facilities for: time of flight detector, laser cooling, electron cooling and mass spectrometry.
> New heavy isotopes 259Db and 265Bh were also synthesized at HIRFL in Lanzhou (China)
In addition to these, ZG Gan has found Barium-136m. 259Db was in 2001, 265Bh was in 2004.
Let’s look up the 2004 paper.
“A new isotope 265Bh was produced and identified at the Sector Focus Cyclotron of the Heavy Ion Research Facility in Lanzhou. This experiment was performed via the reaction of an 243Am target with 168 MeV 26Mg ions. Identification was made by observation of correlated α-particle decays between the new isotope 265Bh and its 261Db and 257Lr daughter nuclei using a set of rotating-wheels system. A total of 8 correlated decay events of 265Bh and 4 decay events of 264Bh were observed. 265Bh decays with a 1 s half-life by emission of α-particles”
“The reaction products recoiled out of the target, and then were stopped in the helium gas which was loaded with the NaCl aerosols. The products attached to the aerosols were continuously swept out of the target chamber with the helium gas, and transported through a 1.2 m length capillary into a collection and measurement chamber.”
So this used the relatively old helium gas, capillary and rotating wheel method.
Let’s look for newer papers by the same chinese author. ZG Gan.
New isotopes of Neptunium – 219, 220, 222, 223, 224 published 2017 to 2020.
These are all neutron-deficient isotopes. Neutron-deficient isotopes of U and Th were also found by the same team.
Let’s look at the 2020 paper.
Primary author L Ma (2020) “Short-Lived α-Emitting Isotope 222Np and the Stability of the N=126 Magic Shell” from https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.125.032502
“α-decay spectroscopy, one of the oldest yet powerful tools in nuclear physics, has been employed generally to identify new (neutron-deficient) heavy isotopes”.
Identification of new isotope using alpha particle energies.
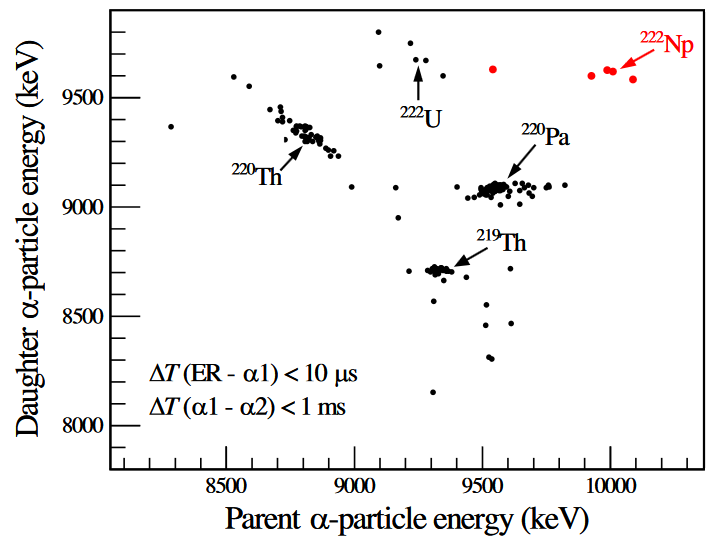
Magic numbers still work in the present day, sometimes.

Date: 28/07/2021 20:44:57
From: mollwollfumble
ID: 1771167
Subject: re: Who discovered the isotopes, and how?
Thread archived.
Time to wrap this thread up, bash it into shape for a report, get ANSTO to co-author it, and submit it to Nature.
Don’t you think?
Date: 28/07/2021 21:09:12
From: The Rev Dodgson
ID: 1771176
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
Thread archived.
Time to wrap this thread up, bash it into shape for a report, get ANSTO to co-author it, and submit it to Nature.
Don’t you think?
Definitely :)
Date: 31/07/2021 22:38:00
From: mollwollfumble
ID: 1772300
Subject: re: Who discovered the isotopes, and how?
The Rev Dodgson said:
mollwollfumble said:
Thread archived.
Time to wrap this thread up, bash it into shape for a report, get ANSTO to co-author it, and submit it to Nature.
Don’t you think?
Definitely :)
Thanks. I’ve finished bashing the report into shape up to the end of Chaper 3 (isotope discoveries 1894 to 1944).
And am now most of the way through Chapter 4 (isotope discoveries up to 1958).
The following real life scientific data gathering exercise would make a good full length Holywood movie. Direct quote from https://en.wikipedia.org/wiki/Ivy_Mike#Scientific_discoveries. “Ivy Mike” is a H-bomb.
“An hour after the H-bomb was detonated, U.S. Air Force pilots took off from Eniwetok Island to fly into the atomic cloud and take samples. Pilots had to monitor extra readouts and displays while “piloting under unusual, dangerous, and difficult conditions” including heat, radiation, unpredictable winds and flying debris. “Red Flight” Leader Virgil Meroney flew into the stem of the explosion first. In five minutes, he had gathered all the samples he could, and exited. Next Bob Hagan and Jimmy Robinson entered the cloud. Robinson hit an area of severe turbulence, spinning out and barely retaining consciousness. He regained control of his plane at 20,000 feet, but the electromagnetic storm had disrupted his instruments. In rain and poor visibility, without working instruments, Hagan and Robinson were unable to find the KB-29 tanker aircraft to refuel. They attempted to return to the field at Enewetak. Hagan, out of fuel, made an extraordinary successful dead-stick landing on the runway. Robinson’s F-84 Thunderjet crashed and sank 3.5 miles short of the island. Robinson’s body was never recovered.”
“Fuel tanks on the airplane’s wings had been modified to scoop up and filter passing debris. The filters from the surviving planes were sealed in lead and sent to Los Alamos, New Mexico for analysis. Radioactive and contaminated with calcium carbonate, the “Mike” samples were extremely difficult to handle. Scientists at Los Alamos found traces in them of isotopes plutonium-246 and plutonium-244. Ghiorso, Thompson and Seaborg obtained half a filter paper from the Ivy Mike test. They were able to detect the existence of the elements einsteinium and fermium, which had been produced by intensely concentrated neutron flux about the detonation site. The discovery was kept secret for several years, but the team was eventually given credit. In 1955 the two new elements 99 and 100 were named in honor of Albert Einstein and Enrico Fermi.”
Date: 2/08/2021 12:10:23
From: mollwollfumble
ID: 1772777
Subject: re: Who discovered the isotopes, and how?
Here we go, this is what I’ve been heading towards the whole time. The when and how of isotope discovery.
It doesn’t include the “who”, but that’s tractable for the early natural actinides, stable isotopes, and bombardment by ions.
It may(?) be tractable for fission products.
It “who” question is certainly not tractable for the discovery of isotopes by bombarding elements with radiation. By 1967, a summary paper listed 10,000 papers by authors who had discovered new isotopes or studied their properties.
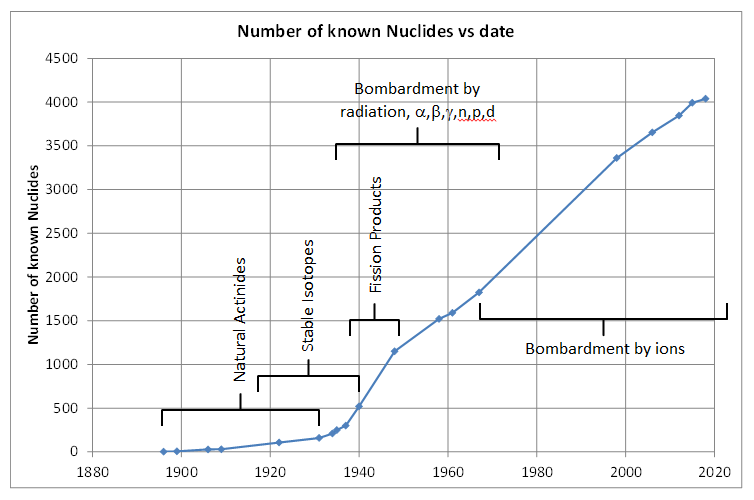
Date: 2/08/2021 12:20:50
From: SCIENCE
ID: 1772780
Subject: re: Who discovered the isotopes, and how?
¿ so what’s next, direct fusion by molecular compression ?
Date: 3/08/2021 19:30:18
From: mollwollfumble
ID: 1773295
Subject: re: Who discovered the isotopes, and how?
SCIENCE said:
¿ so what’s next, direct fusion by molecular compression ?
Good question. That is not such a bad idea. In my book it’s more promising than civilian fusion power.
I have one other suggestion myself. Try to recreate the “r-process” of element creation (found inside supernovas and hydrogen bombs) by bombarding unstable ions with a massive flux of fast neutrons.
One new method that is coming up next is electron bombardment. New isotopes were found sometime between 2018 and 2021 by rapid electron bombardment of transuranics. I haven’t tracked down the who or when yet, but what has been found is already well outside the normal envelope of known isotopes.
I’m at a loss so far to explain some Japanese results from 1990. They found four new isotopes. Their method was to bombard Uranium with neutrons and separate out the results by slowing the products in inert gas and then pumping that gas through a capillary to where the products were deposited on Aluminium. Every single part of that method for finding new isotopes has been around since the year 1937, and must have been carried out very many thousands of times since then.
So why did it take 53 years for these four new isotopes to appear? What new trick have the Japanese added that I’m unaware of?
Date: 5/08/2021 13:42:33
From: mollwollfumble
ID: 1773892
Subject: re: Who discovered the isotopes, and how?
mollwollfumble said:
SCIENCE said:
¿ so what’s next, direct fusion by molecular compression ?
Good question. That is not such a bad idea. In my book it’s more promising than civilian fusion power.
I have one other suggestion myself. Try to recreate the “r-process” of element creation (found inside supernovas and hydrogen bombs) by bombarding unstable ions with a massive flux of fast neutrons.
One new method that is coming up next is electron bombardment. New isotopes were found sometime between 2018 and 2021 by rapid electron bombardment of transuranics. I haven’t tracked down the who or when yet, but what has been found is already well outside the normal envelope of known isotopes.
I’m at a loss so far to explain some Japanese results from 1990. They found four new isotopes. Their method was to bombard Uranium with neutrons and separate out the results by slowing the products in inert gas and then pumping that gas through a capillary to where the products were deposited on Aluminium. Every single part of that method for finding new isotopes has been around since the year 1937, and must have been carried out very many thousands of times since then.
So why did it take 53 years for these four new isotopes to appear? What new trick have the Japanese added that I’m unaware of?
First draft of the report “Who discovered the isotopes, and how?” is completed. Fifteen chapters, 57 pages not counting the table of contents.