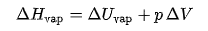Trevtaowillgetyounowhere said:
from the Wikipedia
The enthalpy of vaporization can be written as
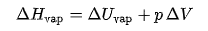
ok got it its magic.
but yeah ok so it simply takes more energy to go from liquid to steam than just heating the liquid from x to y C
Today I learned something I never even noticed or suspected was a thing in over 50 years of living.
Thanks
side question. does the opposite thing happen when you make a liquid turn into a solid? ie. less energy the colder it gets?
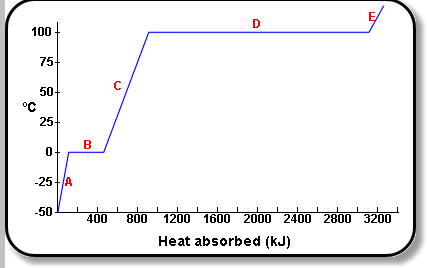
Trev, the above graph shows the energy required for the state changes.
To go from ice to water requires 333 J/g.
To go from water to steam requires 2256 J/g.
Note that going to other way energy is released, so water vapour turning back to water releases 2256 J/g of energy as heat.