wookiemeister said:
mollwollfumble said:
I wish to apologise for being totally wrong in some of the things I said above.
Everything I said about the purpose of tetra-ethyl-lead for instance. Totally wrong.
But not everything.
The 1970s US Clean air act covered oxides of nitrogen, carbon monoxide, unburnt hydrocarbons.
But it did not consider lead in vehicle exhausts to be a sufficiently significant health risk to require emission reductions.
To satisfy the emission requirements of the 1970 clean air act, catalytic converters were added.
Unleaded fuel was initially introduced to stop fouling of catalytic converters by lead, not because leaded fuel was a significant health risk.
I’m learning a lot about how risky lead really is, peeling the research articles off in decades.
For instance, lead is deadly in a large number of different and unrelated ways.
And I have a hypothesis about the origins of anti-lead paranoia.
As it turns out there’s no safe level of lead
You mean like there’s no safe level of nitrogen? Nitrogen causes narcosis you know, even at atmospheric pressure.
——————-
Lead Paranoia
Lead paranoia starts with the use of the phrase “eat lead” in movies from 1955 onwards, and the use of “lead poisoning” as a synonym for being shot in movies from 1933 onwards. There are now nearly 200 movies using the terms “eat lead” or “lead poisoning”, even in comedies including Calamity Jane in 1953 and Hogans Heroes in 1968. Although starting out as a joke, the constant repetition means that some plebs now believe that people being shot die from lead poisoning, and that eating lead has the same symptoms as being shot. There is extra confusion generated by the highly advertised fact that lead poisoning can develop in wildfowl that are shot.
Moving to the real world, in 1898, white lead in paint is stated to be completely non-toxic. There is a disagreement between researchers in 1903-1906. In 1903 it was found that inhaled lead by lead workers was of negligible importance in producing disease, but in 1906 it was judged to be of major importance among lead workers. In the 1906 study, cats were made sick by inhaling and ingesting lead dust. Again there was disagreement, in one part of the experimnent the ingestion of dust by licking fur was found to be a major component of the disease. But this disagrees with the same study showing that direct ingestion is al least 100 times less toxic than inhalation. “One cat was fed 0.8 gr. dry white lead a day for eighteen months without producing any otber symptoms than some loss of weight. Other cats fed similar quantities of white lead daily developed symptoms only when alcohol was administered at the same time.” For an adult human that’s equivalent to eating 12 g of white lead paint per day without producing symptoms. From 1913, the recommended maximum dose is one quarter of that. Symptoms of acute lead poisoning by ingestion, equivalent to 30 g of white lead in one day for an adult human, are vomiting and extreme thirst.
From 1909, “Although lead poisoning of adults in Queensland is unknown, a practitioner will see one of two cases per year of lead poisoning in children”. The “cause has been convincingly demonstrated to be white lead paint on timber”. “The paint degrades in the hot summer, particularly on verandas where children play”. “The first sign of poisoning is a blue line or black dots on the gums.” “The second sign of plumbism is the presence of lead in the urine”. Lead poisoning causes colic, vomiting and loss of appetite, sometimes constipation. This leads to muscle paralysis, particularly of the toes, ankles and wrists, and muscle pains at night. The muscles recover after removal of the poison, over lengths of time ranging from a few weeks to three months. In extreme cases there is paralysis of the diaphragm, which is scarcely noticeable, and finally heart failure”.
(It now occurs to me that if UV stabilisation of paints had been developed earlier, then the degradation to powder would never have occurred and lead white paint would still be considered 100% safe.)
From 1933, “Lead poisoning in infants may follow the prolonged use of lead nipple shields; in Japan poisoning has occurred frequently from the use by the mother of face powder containing lead. In infants and older children the ingestion, over a period of time, of water containing even small amounts of lead may result in intoxication. Recently there was reported an extensive series of cases of lead poisoning following the inhalation of fumes in homes where battery casings were burnt as fuel”.
From 1939, the chemical lead acetate is listed among common causes of poisoning requiring first aid. What is lead acetate used for? In dying textiles, waterproofing and insecticides. It is water soluble, unlike metallic lead which is insoluble in cold and hot water.
From 1943, “lead poisoning occurring in early life usually has a disastrous effect on mental development”
From 1946, “Eleven cases of lead poisoning in the USA were detected among workers manufacturing or mixing tetre-ethyl lead. But there were no cases of lead poisoning or any clinical evidence of lead absorption after 1943”. This improvement is despite increased production, and is attributed to safer work practices.
From 1955, “Lead poisoning is childhood is not a rare disease. Two cases of fatal lead poisoning occurred in Chicago in 1954”. Case 1 seen at ages 2 and 4. At age 2 the symptom was a tendency to fall over. At age 4 vomiting, pain and headache. After death, changes were seen by microscope in the kidneys, liver and bones. In Case 2, death at 1 year 10 months, no history of lead exposure was found. Death was by intercranial pressure and convusions. (PS. ‘No environmental exposure’ rings alarm bells for me, it suggests murder).
Automotive catalytic converters had been developed at least as early as 1936.
From 1973, “Lead, phosphorus, sulfur and several other elements are known to poison catalysts used in reducing carbon monoxide and hydrocarbon emissions from automobile exhausts”.
From 1974, “We have used our equipment for a detailed study of poisoning of a Pt catalyst by lead derived from the fuel”. “Pt-Pd catalyst were exposed to the combustion products of a gasoline containing various concentrations of tetraethyl lead (Motor Mix). The lend concentrations were <0.003, 0.03, and 0.05 g Ph/gal.” With 0.01% lead deposit on the catalyst, hydrocarbon conversion was 60%. By 0.1% lead deposit, that had dropped to 51% conversion and by 1% lead deposit to 42% conversion.
From 1974, “Durability is a critical problem for automotive catalytic converters.” The two main degradation methods are “Poisoning by lead and phosphorus”, and thermal degradation.
This document from 1974 looks good “Potential Dilemma: The Methods of Meeting Automotive Exhaust Emission Standards of the Clean Air Act of 1970” https://ehp.niehs.nih.gov/doi/pdf/10.1289/ehp.748165 . “compliance with this legislation requires an understanding of automotive technology, petroleum refining, atmospheric chemistry and physics, economics, and public health.” “In 1952, demonstrated that smog comes from hydrocarbons and nitric oxides. This study first showed clearly that the automobile, was a public health problem.” “The combustion of leaded gasoline in the spark-ignited automotive engine, in particular was identified as the major mobile source of hydrocarbons, carbon monoxide, nitrogen oxides, lead halide particulates, and other particulate matter.” (Comment by mollwollfumble “Halide?!”, where does halide come from?) “the 1970 Clean Air Act has centered on achieving the exhaust emission standards prescribed by this law for CO, hydrocarbons, and nitrogen oxides. Lead particulate emissions are not regulated.” “The method most often discussed is the use of catalytic mufflers to clean up exhaust emissions and the corresponding removal of the lead antiknock additive since it would poison the catalytic surface of these mufflers.” Other methods …
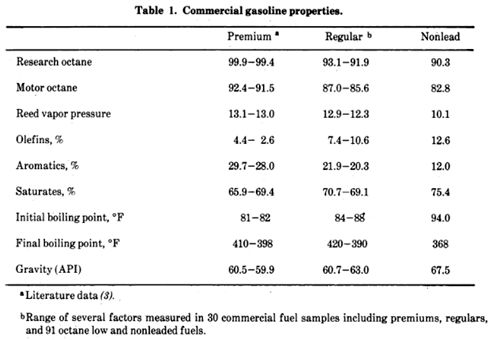
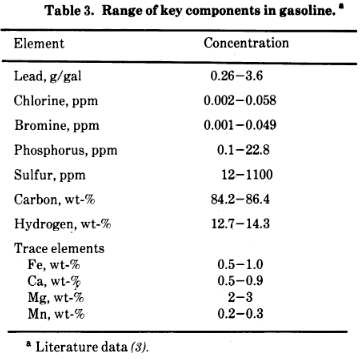
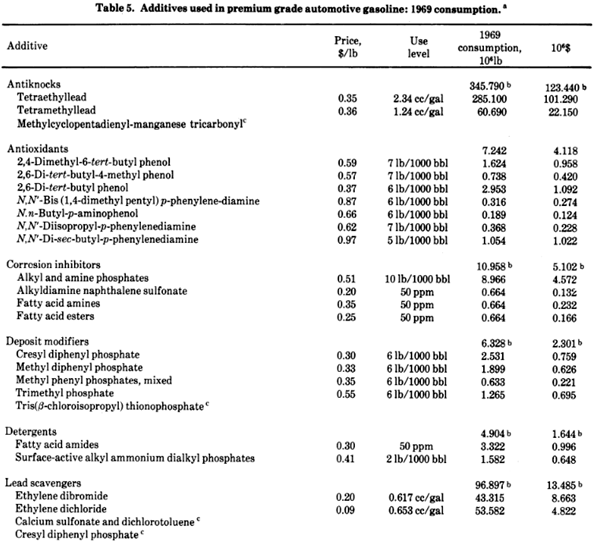
“The gasoline refining and blending industry is highly competitive, with the reasoning for selection of various blends and additives bound up in science, secrecy, and intuition.” “A Task Force Report on Health Intelligence for Fuel and Fuel Additive Registration (1973) sponsored by the EPA average compositions for 30 commercial gasolines.
Premium 1000 ppm tetraethyllead. Regular 400 ppm tetraethyllead. Extra components below:
Antiknock Action and Combustion in Spark-Ignited Engines:
Knock is defined as preignition of the fuel-air mixture during the compression and ignition strokes of the internal combustion engine. In one theory of the mechanism of knock, the compression of a fuel-air mixture to 8-10 atm in fractions of a second results mainly in the oxidation of the saturated paraffinic portion of gasoline (approximately 65-75% by weight) to peroxides, aldehydes, and alcohols.
If this group of parallel reactions is allowed to continue uninhibited, the oxidation process will reach a rate at which ignition and heat release will occur spontaneously. The explosive potential from peroxides has long been recognized by manufacturers and users of ether, since exposure of ether to air results in the formation of peroxides at an appreciable rate. The resulting detonations of this uncontrolled process, called knock, occur before the piston reaches the top of the compression stroke. Since preignition in the compression stroke does not occur at the maximum compression pressure, the knocking condition results in the inefficiency of power derivation and instability in engine operation. Tetraethyllead (TEL) is added to compete for 02 in the fuel-air mixture during the compression stroke. Since TEL oxidizes to PbO at a rate comparable to the rate of oxidation of fuel paraffins, it can suppress the amount of peroxides, aldehydes, and alcohols formed during the compression stroke. In this way, the concentrations of these paraffin oxidation products do not reach the levels required for the rapid heat release process of preignition. Therefore, TEL allows the fuel-air mixture to be compressed to the volume at which optimal power is derived from the design of the engine.
In another theory of the mechanism of knock, … The scavengers for lead antiknock decomposition products are ethylene dichloride and ethylene dibromide. The reaction of these two compounds with lead oxides produces mixed lead halide particulates in the exhaust emissions.