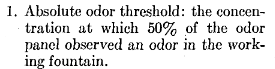dv said:
mollwollfumble said:
dv said:
Benzene has a smell. So do some other non-polar aromatics. (Aromatic here refers to the functional group with six carbons in a ring with effectively 9 bonds shared.)
Yes. I was thinking that. But was wondering if it was a specific property of the pi orbitals that creates the smell. So, why do …
It’s more a property of human olfactory receptor classes. Some of them respond to such rings.
Then you could ask “why did we evolve them” and I would say maybe it helped it identifying some fruits.
Extra comments up front are there are certain chemicals that are odourless yet detectable by smell.
Ethanol is one. I can detect it by its psychoactive effect. Other odourless chemicals detectable by psychoactive effect include oxygen and the narcosis from high pressure gases including nitrogen, neon, argon.
Another chemical that’s odourless but detectable by smell in high concentrations because it has physiological effects such as headaches, is carbon dioxide.
Other extra effect of smell without odour is the (micro-)pain sensations generated by sauna hot air and effervescent aerosols and some nerve gases.
And then, what about odourless chemicals detected by smell through taste?
———-
Is there a list of odourless chemicals anywhere?
O2, N2, CO2, CH4, CO, H2O, He, Ne, Ar, Kr, Xe, Rn, Ethane, Propane.
Is that all? Also CF4, CHF3, CH2F2,
Methanol, Ethanol and Freon are not odourless.
———-
Oh wowee, looky here. https://www.tandfonline.com/doi/pdf/10.1080/00022470.1974.10470005
“Characterization of the Odor Properties of 101 Petrochemicals Using Sensory Methods” from 1974.
It could be extremely useful to be able to identify petrochemicals by smell.
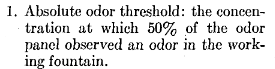

I like the notion of characterising smells by “hedonic tone”.
On the 101 petrochemicals listed, none were odourless. From mildest smell to strongest smell, the mildest in odor order were:
260 Ethylene (ie. identifiable at 0.026% concentration in air, 260 ppm)
260 Ethylene oxide
45 Trichlorotrifluoroethane
22.5 Propylene
20 Acetone
Acetone already has quite a strong smell, so there’s no point here in me going further.
For pleasant hedonic tone at low concentrations, butyl acetate is the most powerful, it has a sweet esther smell to it.
The worst smell in terms of an unpleasant smell at ultra-low concentrations, is ethyl acrylate, smellable at 0.0002 ppm.
There are a lot of smelly petrochemicals that aren’t on the list, benzene and butane for starters.