Pure element toxicity
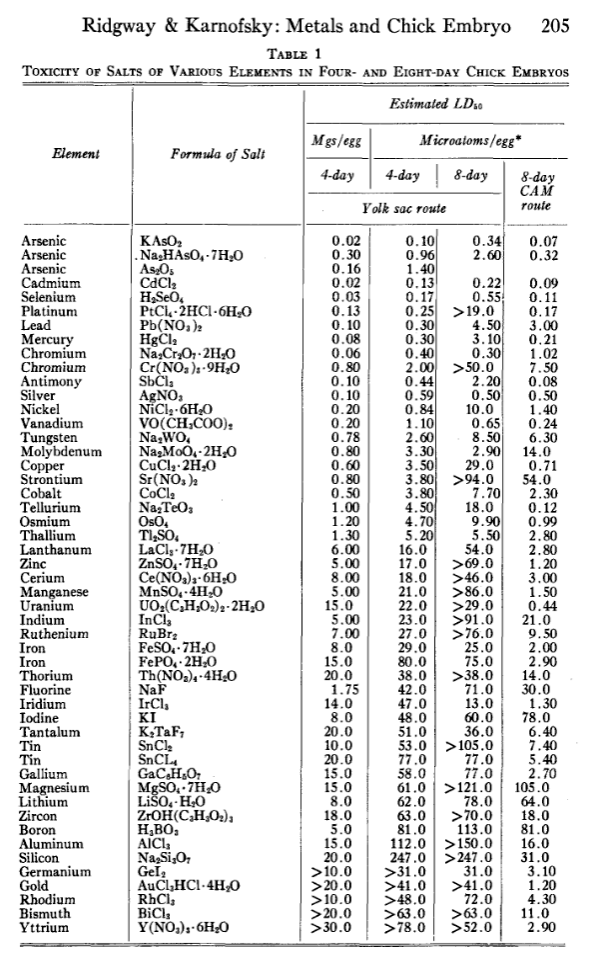
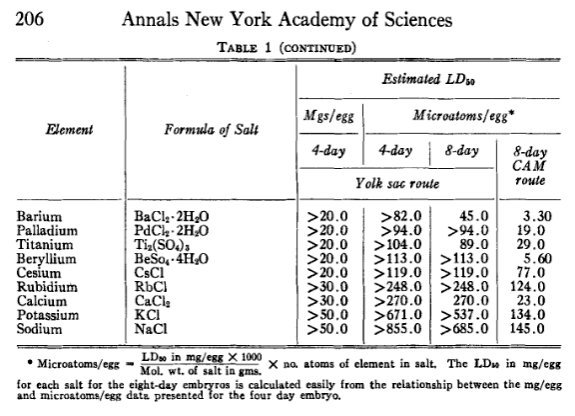
How toxic are pure elements when ingested? Assume that each is ingested as a smooth pill to avoid the physical danger from sharp edges. Or multiple pills so the volume doesn’t exceed what can easily pass through the digestive system.
There ought to a way to compare chemical toxicity. Death would be considered more dangerous than burning, which in turn is more dangerous than diarrhoea, which in turn is more dangerous than chemical absorption due to reactivity, which in turn would be more dangerous than nanoparticle deposition, which in turn is more dangerous than complete inertness.
It should be possible to sort the pure elements in order. From no source other than general knowledge, I’d guess the pure element toxicity would be in the following order, approximately, from safest to least safe. How accurate would I be?
Platinum – reacts only in extreme acid and alkaline liquids
Gold – injected gold nanoparticles can accumulate in the brain
Silver – reacts with sulphur
Iron – safe as a nanoparticle
Tin – safe as a nanoparticle
Zinc – safe as a nanoparticle
Silicon – the physical danger is more severe than the chemical danger
Carbon (as graphite) – reactive
Magnesium – reactive
Aluminium – implicated in dementia
Lead – eventually accumulates
Copper – used in pesticides
Mercury – causes diarrhoea
Iodine – antibiotic, skin irritation
Chromium – used in pesticides
Arsenic – poison, used in pesticides, causes diarrhoea
Thorium – radioactive
Uranium – radioactive
Sulphur – burns the skin
Sodium – burns
Potassium – burns
Thallium – poison
Phosphorus (multiple forms) – really burns
Francium – explosive
Plutonium – radioactive and chemically dangerous
I’ve deliberately placed copper as more dangerous than lead, I suspect that it is because copper is widely used in pesticides and lead isn’t.
What have I missed?