What is so rare it has never been seen directly, because if you could get enough of it together, it would self-vaporize from its own radioactive heat?
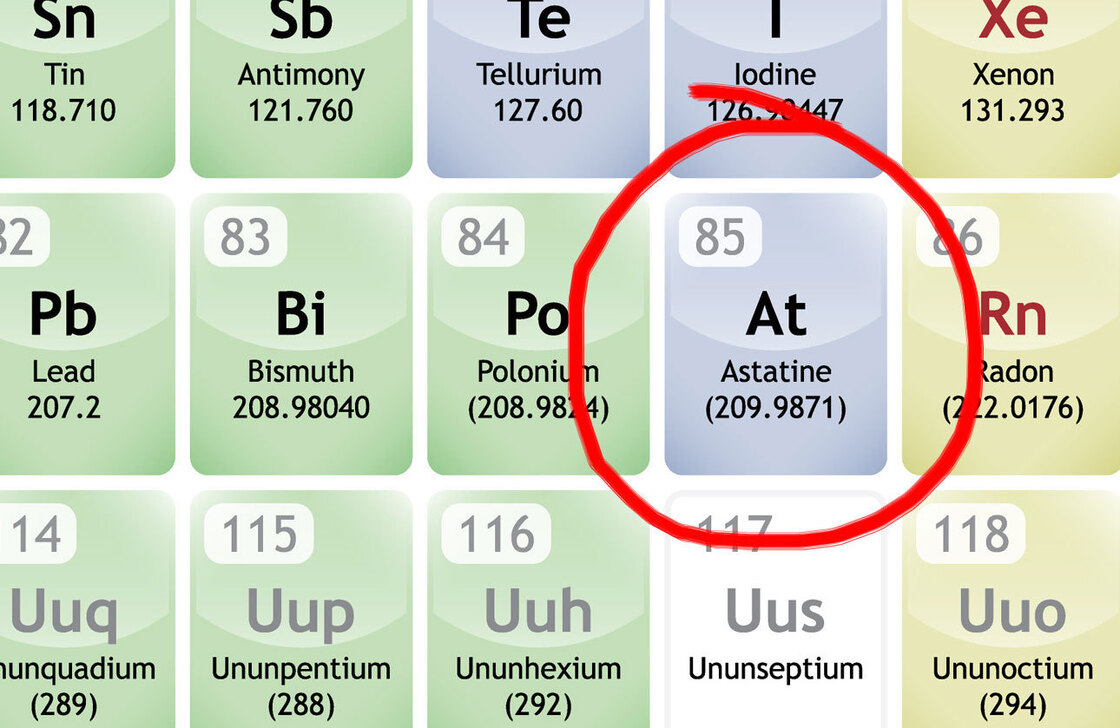
“At” stands for astatine. It is an element with 85 protons packed into its nucleus, thus the atomic number “85” …
The problem is, there’s something about 85 protons in a tight space that nature doesn’t enjoy. Almost as soon as they squeeze together bits of nuclear material get spat out, or get added, and poof!It isn’t astatine any longer.
“This element has a half life of roughly 8 hours, meaning if you could get a clump of it to stay on a table (you can’t), half of it would disintegrate in 8 hours, and then every 8 hours another half would go until in a few days, there’d be no astatine on the table. Its nickname should be “Goodbye!”“
“Some of you will say that another element, francium (atomic number 87), is even more unstable than astatine, and you’re right. Its nickname should be “gone!” As Sam writes, “If you had a million atoms of the longest-lived type of astatine, half of them would disintegrate in 400 minutes. A similar sample of francium would hang on for 20 minutes. Francium is so fragile, it’s basically useless.” Astatine, however, is rarer and can be used to treat thyroid cancers.”
amazing science…
