From Harvard School of Engineering and Applied Sciences:
With the hand of nature trained on a beaker of chemical fluid, the most delicate flower structures have been formed in a Harvard laboratory—and not at the scale of inches, but microns.

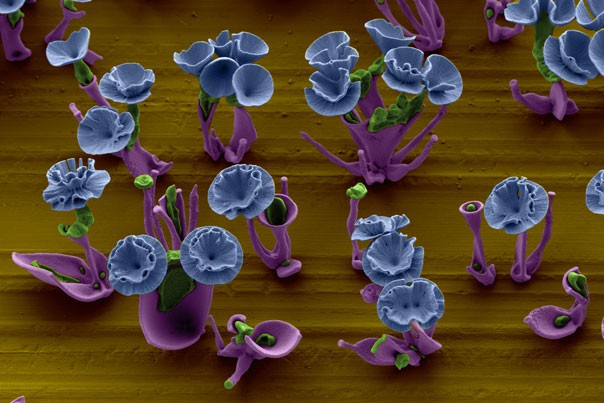
These minuscule sculptures, curved and delicate, don’t resemble the cubic or jagged forms normally associated with crystals, though that’s what they are. Rather, fields of carnations and marigolds seem to bloom from the surface of a submerged glass slide, assembling themselves a molecule at a time.
By simply manipulating chemical gradients in a beaker of fluid, Wim L. Noorduin, a postdoctoral fellow at the Harvard School of Engineering and Applied Sciences (SEAS) and lead author of a paper appearing on the cover of the May 17 issue of Science, has found that he can control the growth behavior of these crystals to create precisely tailored structures.
“For at least 200 years, people have been intrigued by how complex shapes could have evolved in nature. This work helps to demonstrate what’s possible just through environmental, chemical changes,” says Noorduin.
The precipitation of the crystals depends on a reaction of compounds that are diffusing through a liquid solution. The crystals grow toward or away from certain chemical gradients as the pH of the reaction shifts back and forth. The conditions of the reaction dictate whether the structure resembles broad, radiating leaves, a thin stem, or a rosette of petals.
It is not unusual for chemical gradients to influence growth in nature; for example, delicately curved marine shells form from calcium carbonate under water, and gradients of signaling molecules in a human embryo help set up the plan for the body. Similarly, Harvard biologist Howard Berg has shown that bacteria living in colonies can sense and react to plumes of chemicals from one another, which causes them to grow, as a colony, into intricate geometric patterns.
Replicating this type of effect in the laboratory was a matter of identifying a suitable chemical reaction and testing, again and again, how variables like the pH, temperature, and exposure to air might affect the nanoscale structures.
Full report: https://www.seas.harvard.edu/news/2013/05/beautiful-flowers-self-assemble-beaker