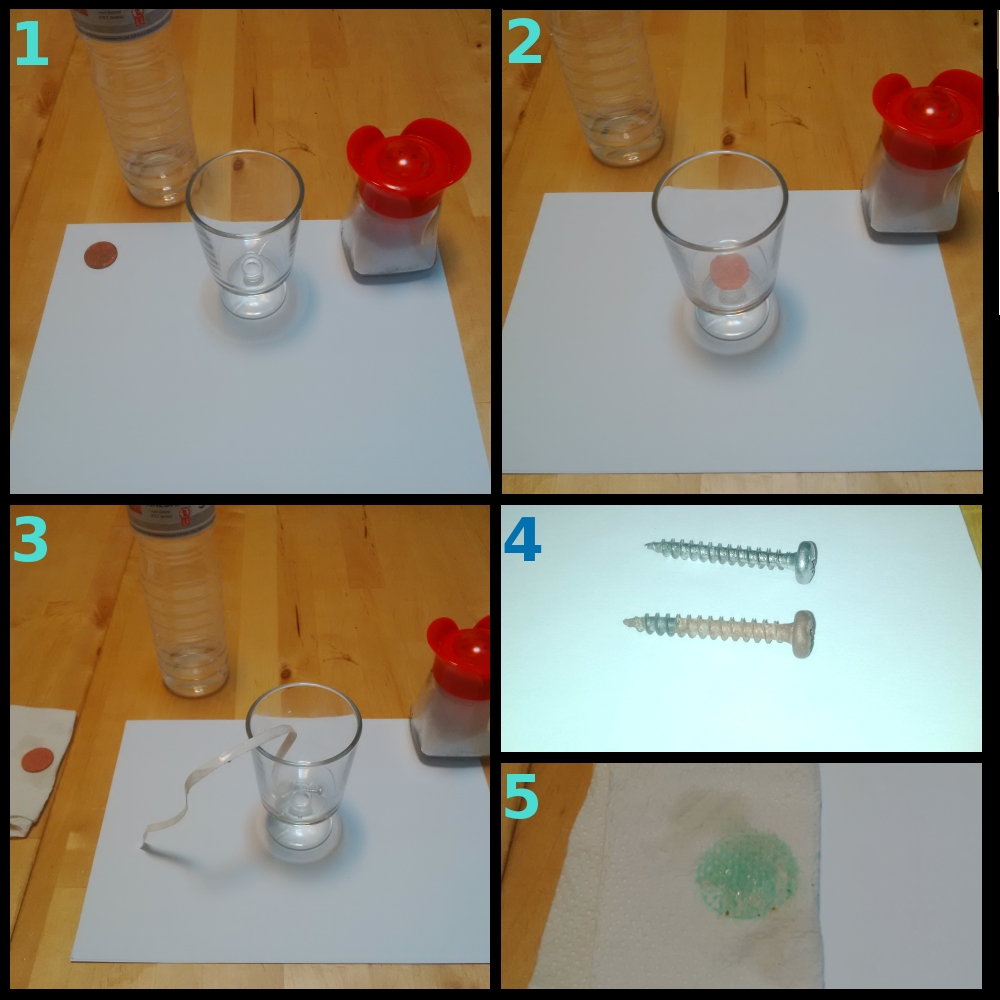
Here’s another little household chemistry experiment that requires no special gear, which I am using to teach about displacement reactions and solubility at home.
Figure 1/ You just need a steel nail or screw, old tarnish copper coin(s), salt and vinegar.
Figure 2/
Mix the salt and vinegar in a container. This produces a dilute hydrochloric acid solution.
CH 3 COOH (aq) + NaCl(aq) -> CH 3 COONa + HCl(aq)
Put the coin in the container and swirl it around. The patina will be dissolved. (NB do not do this with valuable coins as this actually damages them a bit.) Copper (II) hydroxide is not in itself very soluble in water but copper (II) chloride is.
Cu(OH) 2 (s) + 2HCl(aq) -> CuCl 2 (aq) + 2H 2 O
Figure 3/ Place the still-wet coin on some kitchen paper. Put the nail or screw in the solution, partially submerged. Leave that for a few hours.
Figure 4/ Iron is more reactive than copper, so a displacement reaction takes place: iron enters the solution and copper is deposited, coating the nail or screw. Here we see the coated screw compared to one in its original condition.
CuCl 2 (aq) + Fe(s) -> Cu(s) + FeCl 2 (aq)
Figure 5/ More green copper hydroxide has formed on the acid-wet coin, shown here as a stain on the kitchen paper.