Date: 29/09/2015 06:41:03
From: mollwollfumble
ID: 781523
Subject: Water on Mars, really, this time.
OK. I have egg on face. I’ve been a water on Mars denier. My argument has been that no liquid water ever flows on Mars because the atmospheric pressure on Mars is everywhere below the triple point of water, below which no liquid water can exist. True, the presence of salts can reduce the melting point of water, but I’ve said that while possible, it’s very unlikely to actually happen.
Now new news from NASA is that liquid water definitely flows on Mars in the present day.
http://www.nasa.gov/press-release/nasa-confirms-evidence-that-liquid-water-flows-on-today-s-mars
“Recurring slope lineae (RSL)” are “darkish streaks appear to ebb and flow over time. They darken and appear to flow down steep slopes during warm seasons, and then fade in cooler seasons. They appear in several locations on Mars when temperatures are above minus 10 degrees Fahrenheit (minus 23 Celsius), and disappear at colder times.” They contain “hydrated salts only when the seasonal features were widest, which suggests that either the dark streaks themselves or a process that forms them is the source of the hydration”. “The hydrated salts would lower the freezing point of a liquid brine”. “It’s likely a shallow subsurface flow, with enough water wicking to the surface to explain the darkening.”
RSL’s were found “using images from the Mars Reconnaissance Orbiter’s (MRO) High Resolution Imaging Science Experiment (HiRISE). HiRISE observations now have documented RSL at dozens of sites on Mars. The new study pairs HiRISE observations with mineral mapping by MRO’s Compact Reconnaissance Imaging Spectrometer for Mars (CRISM).”
“The hydrated salts most consistent with the chemical signatures are likely a mixture of magnesium perchlorate, magnesium chlorate and sodium perchlorate. Some perchlorates have been shown to keep liquids from freezing even when conditions are as cold as minus 94 degrees Fahrenheit (minus 70 Celsius). On Earth, naturally produced perchlorates are concentrated in deserts, and some types of perchlorates can be used as rocket propellant. Perchlorates have previously been seen on Mars. NASA’s Phoenix lander and Curiosity rover both found them in the planet’s soil, and some scientists believe that the Viking missions in the 1970s measured signatures of these salts. However, this study of RSL detected perchlorates, now in hydrated form, in different areas than those explored by the landers. This also is the first time perchlorates have been identified from orbit.”
Date: 29/09/2015 06:52:07
From: stumpy_seahorse
ID: 781525
Subject: re: Water on Mars, really, this time.
running water on Mars eh?…
the sad thing is, they’ve probably got a better chance of getting a plumber than Bubblecar…
Date: 29/09/2015 07:24:56
From: mollwollfumble
ID: 781530
Subject: re: Water on Mars, really, this time.
mollwollfumble said:
OK. I have egg on face. I’ve been a water on Mars denier. My argument has been that no liquid water ever flows on Mars because the atmospheric pressure on Mars is everywhere below the triple point of water, below which no liquid water can exist. True, the presence of salts can reduce the melting point of water, but I’ve said that while possible, it’s very unlikely to actually happen.
Now new news from NASA is that liquid water definitely flows on Mars in the present day.
http://www.nasa.gov/press-release/nasa-confirms-evidence-that-liquid-water-flows-on-today-s-mars
“Recurring slope lineae (RSL)” are “darkish streaks appear to ebb and flow over time. They darken and appear to flow down steep slopes during warm seasons, and then fade in cooler seasons. They appear in several locations on Mars when temperatures are above minus 10 degrees Fahrenheit (minus 23 Celsius), and disappear at colder times.” They contain “hydrated salts only when the seasonal features were widest, which suggests that either the dark streaks themselves or a process that forms them is the source of the hydration”. “The hydrated salts would lower the freezing point of a liquid brine”. “It’s likely a shallow subsurface flow, with enough water wicking to the surface to explain the darkening.”
RSL’s were found “using images from the Mars Reconnaissance Orbiter’s (MRO) High Resolution Imaging Science Experiment (HiRISE). HiRISE observations now have documented RSL at dozens of sites on Mars. The new study pairs HiRISE observations with mineral mapping by MRO’s Compact Reconnaissance Imaging Spectrometer for Mars (CRISM).”
“The hydrated salts most consistent with the chemical signatures are likely a mixture of magnesium perchlorate, magnesium chlorate and sodium perchlorate. Some perchlorates have been shown to keep liquids from freezing even when conditions are as cold as minus 94 degrees Fahrenheit (minus 70 Celsius). On Earth, naturally produced perchlorates are concentrated in deserts, and some types of perchlorates can be used as rocket propellant. Perchlorates have previously been seen on Mars. NASA’s Phoenix lander and Curiosity rover both found them in the planet’s soil, and some scientists believe that the Viking missions in the 1970s measured signatures of these salts. However, this study of RSL detected perchlorates, now in hydrated form, in different areas than those explored by the landers. This also is the first time perchlorates have been identified from orbit.”
So, reversing my “water on Mars denier” stance, and turning to the scientific literature, I find
The three types of liquid water in the surface of present Mars
“Thermodynamics teaches that pure liquid bulk water cannot stably exist on the surface
of Mars. However, it is shown by thermodynamic arguments that liquid water can exist, at least
temporarily, in the upper surface of Mars, in form of: … (b) undercooled liquid water in cryo-brines.
“Temperatures on Mars at temperate and low latitudes would
permit, at least temporarily, the existence of liquid water on
the surface of present Mars; however, the total atmospheric
pressure (global average 600 mPa) on the majority of the
surface of Mars is below the triple point of water at 273.16 K
and 611.73 Pa. Thus, only at areas of low elevation with a
resulting local higher atmospheric pressure, there are appropriate
thermodynamical conditions that could be given to,
at least temporarily, having liquid water on the surface.
However, a free surface of water will (at 0 ˚C) effectively
evaporate a mass of 3.207*10^4 kg/m^2s of water (Taylor
et al. 2006). To have a steady lake on the surface of present
Mars, this amount of water would be needed to be steadily
delivered from a continuous subsurface source, and this already
over ‘geologic time-scales’. These sources are not
known to exist.”
Even so, nothing in either of the above sources mentions the critical data: by how much, if at all, does the presence of perchlorates decrease the pressure of the triple point of water? Let’s try again, this time with Stability of liquid saline water on present day Mars
“We found that dehydrated sodium perchlorate at the atmospheric conditions of Phoenix landing site (CO2 at 700 Pa) increases its volume and changes phase by deliquescing at temperatures as low as 225 K. … after roughly 5 minutes, at 240–250 K only the liquid phase is present on the sample”.
This article still doesn’t mention any depression of the pressure at the triple point. But at least the pressure is representative of a real location on Mars. PS. This article looks like the physical experiment was thrown together in a few minutes and completed in an afternoon.
There is only one more technical article on the topic in English. Possible physical and thermodynamical evidence for liquid water at the Phoenix landing site
“The water vapor pressure at the triple point of water (∼600 Pa) is below the present-day atmospheric pressure on the lowest regions of Mars such as the Phoenix landing site (∼700–800 Pa), and the low surface temperature (∼180–250 K) and dry air of these regions inhibits the presence of pure liquid water near the surface” .
Nope, this article still doesn’t mention any depression of the pressure at the triple point.
Date: 29/09/2015 09:09:10
From: mollwollfumble
ID: 781561
Subject: re: Water on Mars, really, this time.
> Nope, this article still doesn’t mention any depression of the pressure at the triple point.
Searching the web for any reference to depression of the pressure at the triple point by any dissolved salt.
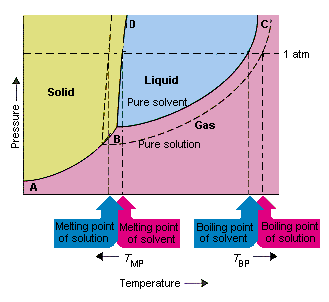
comes from http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch15/colligative.php
Raoult’s law – the vapour pressure above a solution is the product of the vapour pressure above the solute and the mole fraction of solute – is a good first step, but fails to include the effect of freezing point depression at low pressures.
Let’s calculate the approximate reduction in triple point pressure from Raoult’s Law under conditions that may be found on Mars. The eutectic point for magnesium perchlorate in water is 44% by weight. That’s a mole fraction of solvent of 94.0%. So the triple point is reduced from 0.6117 kPa to 0.585 kPa. Is that a significant difference?
The atmospheric pressure on Mars averages … um, different web sources give radically different figures. The standard Mars atmosphere tables from Mars Global Surveyor are not publicly available :-(
Wikipedia has 0.6 kPa.
Mars Pathfinder gives ~0.67 kPa see graph below.

866 days of the Curiosity Rover gives a sizable variation from 0.75 to 0.92 kPa

I suppose I should have factored in temeprature and pressure together, rather than concentrating on the triple point.
Date: 29/09/2015 16:57:56
From: Michael V
ID: 781712
Subject: re: Water on Mars, really, this time.
Whilst surface water might be a good thing if one is looking for life, chlorates and perchlorates dissolved in it are not; they are powerful oxidisers.
Date: 29/09/2015 17:00:58
From: Bubblecar
ID: 781714
Subject: re: Water on Mars, really, this time.
Michael V said:
Whilst surface water might be a good thing if one is looking for life, chlorates and perchlorates dissolved in it are not; they are powerful oxidisers.
And we’ve known since Viking that the ordinary Martian dust is extremely oxidising.
Date: 29/09/2015 17:12:35
From: CrazyNeutrino
ID: 781717
Subject: re: Water on Mars, really, this time.
Bubblecar said:
Michael V said:
Whilst surface water might be a good thing if one is looking for life, chlorates and perchlorates dissolved in it are not; they are powerful oxidisers.
And we’ve known since Viking that the ordinary Martian dust is extremely oxidising.
I wonder how much water is contained under the surface of Mars?
Date: 29/09/2015 17:57:50
From: dv
ID: 781739
Subject: re: Water on Mars, really, this time.
moll: madd props for being candid.
Date: 29/09/2015 18:11:45
From: dv
ID: 781758
Subject: re: Water on Mars, really, this time.
Seems weird that the rivulets start at the very top of the crater rims.
Date: 29/09/2015 18:15:54
From: Bubblecar
ID: 781762
Subject: re: Water on Mars, really, this time.
dv said:
Seems weird that the rivulets start at the very top of the crater rims.
Presumably draining from a wide area and it’s warmer at the top than further down.
Date: 29/09/2015 18:17:48
From: dv
ID: 781764
Subject: re: Water on Mars, really, this time.
Bubblecar said:
dv said:
Seems weird that the rivulets start at the very top of the crater rims.
Presumably draining from a wide area and it’s warmer at the top than further down.
Nah I mean these crater rims are elevated, they are the highest parts locally, nothing can drain to them.
Date: 29/09/2015 18:18:53
From: Bubblecar
ID: 781766
Subject: re: Water on Mars, really, this time.
dv said:
Bubblecar said:
dv said:
Seems weird that the rivulets start at the very top of the crater rims.
Presumably draining from a wide area and it’s warmer at the top than further down.
Nah I mean these crater rims are elevated, they are the highest parts locally, nothing can drain to them.
Fair enough, you should have posted a diagram.
Date: 29/09/2015 18:21:55
From: Bubblecar
ID: 781768
Subject: re: Water on Mars, really, this time.
Maybe it requires that degree of steepness for gravity to get the sludge to flow.
Date: 29/09/2015 18:24:35
From: dv
ID: 781772
Subject: re: Water on Mars, really, this time.
Bubblecar said:
dv said:
Bubblecar said:
Presumably draining from a wide area and it’s warmer at the top than further down.
Nah I mean these crater rims are elevated, they are the highest parts locally, nothing can drain to them.
Fair enough, you should have posted a diagram.
My apols
Date: 29/09/2015 18:30:15
From: CrazyNeutrino
ID: 781775
Subject: re: Water on Mars, really, this time.
perhaps the sun heats up the ground during the summer months and the ground starts leaking water
something like the way the sun creates vents on comets but mars has an atmosphere so the temperature and pressure are all part of the right conditions?
Date: 29/09/2015 18:48:15
From: dv
ID: 781790
Subject: re: Water on Mars, really, this time.
I was a little surprised that Planetary Protection Protocols had been loosened up since the Viking days.
Date: 29/09/2015 19:09:58
From: mollwollfumble
ID: 781801
Subject: re: Water on Mars, really, this time.
Michael V said:
Whilst surface water might be a good thing if one is looking for life, chlorates and perchlorates dissolved in it are not; they are powerful oxidisers.
Absolutely agree. No question about it. The fact that Mars has less carbon on its surface than the Moon does is also not a good sign for life.
I’ve got a new piece of trivia for you. I haven’t checked it thoroughly, but I think I’ll find that the nearest astronomical object to Earth capable of supporting life-as-we-know-it on its surface is WISE 0535-7500. Why? Because WISE 0350-5658 is too hot and WISE 0855−0714 is too cold ;-)
CrazyNeutrino said:
I wonder how much water is contained under the surface of Mars?
perhaps the sun heats up the ground during the summer months and the ground starts leaking water. something like the way the sun creates vents on comets but mars has an atmosphere so the temperature and pressure are all part of the right conditions?
Underground as ice, plenty. As hydrated minerals, even more. But I can’t tell you what fraction.
As for season. Yes. Both temperature and pressure. At low pressures it all vapourises instead of liquefies. Pressure is a minimum on Mars in early spring and a maximum in early summer and early winter.
Date: 29/09/2015 20:46:42
From: PermeateFree
ID: 781826
Subject: re: Water on Mars, really, this time.
CrazyNeutrino said:
perhaps the sun heats up the ground during the summer months and the ground starts leaking water
something like the way the sun creates vents on comets but mars has an atmosphere so the temperature and pressure are all part of the right conditions?
Sounds like hydrothermal activity, which on earth the water can be very hot, which may also account for the 100 metre run before petering out.
Date: 29/09/2015 21:25:58
From: dv
ID: 781850
Subject: re: Water on Mars, really, this time.
One other thing that was mentioned in the press conference was that the 2020 Mars mission will include the first ISRU experiment, which will convert carbon dioxide into carbon monoxide and oxygen. This could in future be used to generate oxygen to support a human mission, or in the short term to produce bipropellent for a sample return mission (CO and O2 make a decent rocketry bipropellent).
Date: 29/09/2015 23:34:46
From: mollwollfumble
ID: 781914
Subject: re: Water on Mars, really, this time.
dv said:
One other thing that was mentioned in the press conference was that the 2020 Mars mission will include the first ISRU experiment, which will convert carbon dioxide into carbon monoxide and oxygen. This could in future be used to generate oxygen to support a human mission, or in the short term to produce bipropellent for a sample return mission (CO and O2 make a decent rocketry bipropellent).
Was that copied from the (rejected) Mars Express proposal? I remember that the Mars Express proposal was to manufacture rocket fuel on Mars for the return journey.
Date: 29/09/2015 23:46:04
From: dv
ID: 781919
Subject: re: Water on Mars, really, this time.
mollwollfumble said:
dv said:
One other thing that was mentioned in the press conference was that the 2020 Mars mission will include the first ISRU experiment, which will convert carbon dioxide into carbon monoxide and oxygen. This could in future be used to generate oxygen to support a human mission, or in the short term to produce bipropellent for a sample return mission (CO and O2 make a decent rocketry bipropellent).
Was that copied from the (rejected) Mars Express proposal? I remember that the Mars Express proposal was to manufacture rocket fuel on Mars for the return journey.
I think perhaps you mean Mars Direct, which did involve ISRU for the return rocket trip (using local water and carbon dioxide to produce the methane and oxygen). (Mars Express is the ongoing ESA mission).
Date: 29/09/2015 23:55:32
From: Michael V
ID: 781921
Subject: re: Water on Mars, really, this time.
dv said:
mollwollfumble said:
dv said:
One other thing that was mentioned in the press conference was that the 2020 Mars mission will include the first ISRU experiment, which will convert carbon dioxide into carbon monoxide and oxygen. This could in future be used to generate oxygen to support a human mission, or in the short term to produce bipropellent for a sample return mission (CO and O2 make a decent rocketry bipropellent).
Was that copied from the (rejected) Mars Express proposal? I remember that the Mars Express proposal was to manufacture rocket fuel on Mars for the return journey.
I think perhaps you mean Mars Direct, which did involve ISRU for the return rocket trip (using local water and carbon dioxide to produce the methane and oxygen). (Mars Express is the ongoing ESA mission).
I’d reckon those perchlorates and chlorates would be useful oxidisers for rocket propellants.
Date: 30/09/2015 01:46:42
From: dv
ID: 781932
Subject: re: Water on Mars, really, this time.
Interesting fact:
The Mars Reconnaissance Orbiter, whose HiRISE imager took most of the high resolution images that have been used in this study, has sent back more data than all the other interplanetary missions in history combined, about 250 terabytes of compressed data so far in 10 years of operation.
Although its nominal resolution is 0.3 metres, it won’t be mapping the entire planet at that resolution: if it did, that would end up being about 900 terabytes compressed even in monochrome.
Date: 30/09/2015 08:37:29
From: diddly-squat
ID: 781946
Subject: re: Water on Mars, really, this time.
dv said:
Interesting fact:
The Mars Reconnaissance Orbiter, whose HiRISE imager took most of the high resolution images that have been used in this study, has sent back more data than all the other interplanetary missions in history combined, about 250 terabytes of compressed data so far in 10 years of operation.
Although its nominal resolution is 0.3 metres, it won’t be mapping the entire planet at that resolution: if it did, that would end up being about 900 terabytes compressed even in monochrome.
that’s one impressive flickr account
Date: 30/09/2015 09:15:04
From: The Rev Dodgson
ID: 781962
Subject: re: Water on Mars, really, this time.
dv said:
Interesting fact:
Although its nominal resolution is 0.3 metres, it won’t be mapping the entire planet at that resolution: if it did, that would end up being about 900 terabytes compressed even in monochrome.
So 900 cheap hard drives from Office Works. About $90,000 or less than half that (probably) if you bought them in the USA.
Date: 30/09/2015 09:21:12
From: diddly-squat
ID: 781964
Subject: re: Water on Mars, really, this time.
The Rev Dodgson said:
dv said:
Interesting fact:
Although its nominal resolution is 0.3 metres, it won’t be mapping the entire planet at that resolution: if it did, that would end up being about 900 terabytes compressed even in monochrome.
So 900 cheap hard drives from Office Works. About $90,000 or less than half that (probably) if you bought them in the USA.
and one hell of a mobile data plan
Date: 30/09/2015 19:45:58
From: party_pants
ID: 782146
Subject: re: Water on Mars, really, this time.
It’s a god-awful small affair…
Date: 30/09/2015 21:32:26
From: wookiemeister
ID: 782173
Subject: re: Water on Mars, really, this time.
mars never underwent any change to its atmosphere composition – that’s how they know life never happened on mars
when life happens it changes the composition of the atmosphere
from the rocks and the atmosphere found in them and comparing it to present day there’s very little to no difference for quite some time
Date: 30/09/2015 22:29:59
From: dv
ID: 782209
Subject: re: Water on Mars, really, this time.
wookiemeister said:
mars never underwent any change to its atmosphere composition – that’s how they know life never happened on mars
when life happens it changes the composition of the atmosphere
from the rocks and the atmosphere found in them and comparing it to present day there’s very little to no difference for quite some time
You claim to know a lot more about the composition of Mars’s early atmosphere than anyone else does. I didn’t even know you have a space agency.
In any case: no.
Life did not change the atmosphere appreciably until oxygenic photosynthesis thrived, some 2.3 billion years ago: some 1.5 billion years after life arose.
Basically all we can say from the fact that Mars’s atmosphere is not now oxygenated is that oxygenic photosynthesis is not widespread now.
Date: 30/09/2015 22:36:18
From: wookiemeister
ID: 782220
Subject: re: Water on Mars, really, this time.
dv said:
wookiemeister said:
mars never underwent any change to its atmosphere composition – that’s how they know life never happened on mars
when life happens it changes the composition of the atmosphere
from the rocks and the atmosphere found in them and comparing it to present day there’s very little to no difference for quite some time
You claim to know a lot more about the composition of Mars’s early atmosphere than anyone else does. I didn’t even know you have a space agency.
In any case: no.
Life did not change the atmosphere appreciably until oxygenic photosynthesis thrived, some 2.3 billion years ago: some 1.5 billion years after life arose.
Basically all we can say from the fact that Mars’s atmosphere is not now oxygenated is that oxygenic photosynthesis is not widespread now.
no i wen down to my local astronomy club and listened to a very learned fellow give a talk about what is already a known and accepted viewpoint
Date: 30/09/2015 22:54:19
From: dv
ID: 782235
Subject: re: Water on Mars, really, this time.
wookiemeister said:
dv said:
wookiemeister said:
mars never underwent any change to its atmosphere composition – that’s how they know life never happened on mars
when life happens it changes the composition of the atmosphere
from the rocks and the atmosphere found in them and comparing it to present day there’s very little to no difference for quite some time
You claim to know a lot more about the composition of Mars’s early atmosphere than anyone else does. I didn’t even know you have a space agency.
In any case: no.
Life did not change the atmosphere appreciably until oxygenic photosynthesis thrived, some 2.3 billion years ago: some 1.5 billion years after life arose.
Basically all we can say from the fact that Mars’s atmosphere is not now oxygenated is that oxygenic photosynthesis is not widespread now.
no i wen down to my local astronomy club and listened to a very learned fellow give a talk about what is already a known and accepted viewpoint
I don’t give a fuck where you went, wookie. It should be obvious to you that no one in the industry thinks that there is any good evidence that life never arose on Mars, and if you knew anything about the subject matter, you’d have understood the point I just made.
You really think the entire planetary science community missed some basic point like that?
Date: 30/09/2015 22:55:31
From: wookiemeister
ID: 782237
Subject: re: Water on Mars, really, this time.
dv said:
wookiemeister said:
dv said:
You claim to know a lot more about the composition of Mars’s early atmosphere than anyone else does. I didn’t even know you have a space agency.
In any case: no.
Life did not change the atmosphere appreciably until oxygenic photosynthesis thrived, some 2.3 billion years ago: some 1.5 billion years after life arose.
Basically all we can say from the fact that Mars’s atmosphere is not now oxygenated is that oxygenic photosynthesis is not widespread now.
no i wen down to my local astronomy club and listened to a very learned fellow give a talk about what is already a known and accepted viewpoint
I don’t give a fuck where you went, wookie. It should be obvious to you that no one in the industry thinks that there is any good evidence that life never arose on Mars, and if you knew anything about the subject matter, you’d have understood the point I just made.
You really think the entire planetary science community missed some basic point like that?
oh well we’ll see DV let me know what the results are
Date: 2/10/2015 09:08:37
From: mollwollfumble
ID: 782791
Subject: re: Water on Mars, really, this time.
> Life did not change the atmosphere appreciably until oxygenic photosynthesis thrived, some 2.3 billion years ago: some 1.5 billion years after life arose.
Perhaps not, but but perhaps we just don’t know enough about that early time yet. Life was already changing the Earth’s mineralogy long before that. The most complicated natural minerals are those that could not have formed without the presence of living organisms. Those, although rare in abundance, are very numerous in variety.
Date: 2/10/2015 12:31:53
From: mollwollfumble
ID: 782899
Subject: re: Water on Mars, really, this time.
What water flowing on Mars really looks like.
RSL image from apod

Date: 2/10/2015 12:34:00
From: dv
ID: 782902
Subject: re: Water on Mars, really, this time.
I’ve sometimes quenched my thirst at the RSL
Date: 2/10/2015 12:53:31
From: Bubblecar
ID: 782908
Subject: re: Water on Mars, really, this time.
I wish they’d STFU about LIFE.



