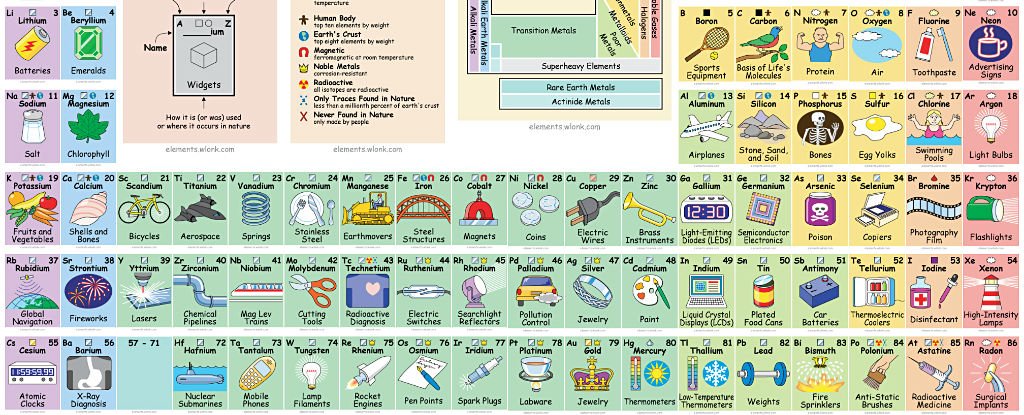
Ian said:
Which is the least useful of the elements on this table?
Excellent question. I’m going to approach it slowly by distinguishing between “least used” and “least potentially useful”.
There’s also the balance between availability and use. Low availability can match low use.
There are also trade-offs, where one element can substitute for another.
To make things easier, I’m going to group elements together by use.
Food supplements – trace minerals, for humans and for other organisms.
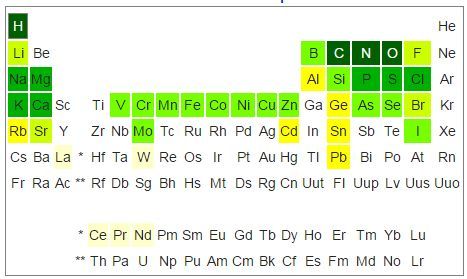
High melting point elements, from highest down.
Carbon, Tungsten, Thenium, Osmium, Tantalum, Molybdenumm, Niobium, Iridium, Ruthenium, Hafnium, Boron, Rhodium, Vanadium, Chromium, Zirconium
Elements that have been used in making superconductors (incomplete list, alphabetical order by symbol).
Aluminium, Cadmium, Carbon, Gallium, Hafnium, Mercury, Indium, Iridium, Lanthanum, Molybdenum, Niobium, Osmium, Palladium, Lead, Rhenium, Ruthenium, Silicon, Tin, Tantalum, Technetium, Thorium, Titanium, Thulium, Uranium, Vanadium, Tungsten, Zinc, Zirconium
Barium, Calcium, Lithium, Potassium, Sodium, Rubidium, Strontium, Ytterbium, Iron, Boron, Indium, Nitrogen, Oxygen, Lanthanum, Magnesium, Germanium.
List of common alloys by base metal (alphabetical order)
Aluminium, Bismuth, Chromium, Cobalt, Copper, Gallium, Gold, Indium, Iron, Lead, Magnesium, Mercury, Nickel, Plutonium, Potassium, Cerium Terbium, Rhodium, Samarium, Scandium, Silver, Sodium, Titanium, Tin, Uranium, Zinc, Zirconium
Elements in the most common low-alloy steels (random order)
Carbon, Manganese, Molybdenum, Silicon, Chromium, Nickel, Boron, Vanadium, Tungsten, Copper
Radioactive medical applications. See web link for an excellent description of what medical applications each is used for.
https://www.isotopes.gov/outreach/med_isotopes.html
Actinium, Americium, Arsenic, Astatine, Gold, Boron, Beryllium, Bismuth, Boron, Carbon, Cadmium, Cerium, Californium, Cobalt, Chromium, Caesium, Copper, Dyprosium, Europium, Fluorine, Iron, Gallium, Gadolinium, Germanium, Hydrogen, Iodine, Indium, Iridium, Krypton, Lutetium, Manganese, Molybdenum, Nitrogen, Niobium, Oxygen, Osmium, Phosphorus, Lead, Palladium, Plutonium, Radon, Rubidium, Rhenium, Rutherfordium, Silicon, Samarium, Tin, Strontium, Tantalum, Terbium, Technetium, Thorium, Thallium, Thulium, Tungsten, Xenon, Yttrium, Ytterbium, Zinc, Zirconium.
So to answer the above question:
> Which is the least useful of the elements on this table?
Well, Helium, Neon, Argon haven’t appeared in any of the above lists yet. LOL. Yes, they are useful.
Beyond that, Promethium, Francium, then you have to go highly radioactive to Berkelium, Einsteinium to find anything not currently useful.
So, to answer your question, Promethium and Francium are the least useful elements, so far.