CrazyNeutrino said:
It’s official: Your Periodic Table is now obsolete
Get ready to ring in 2017 with a brand new Periodic Table, because four more elements have officially been added to the seventh row: nihonium (Nh), moscovium (Mc), tennessine (Ts), and oganesson (Og).
More…
> When and where were these found?
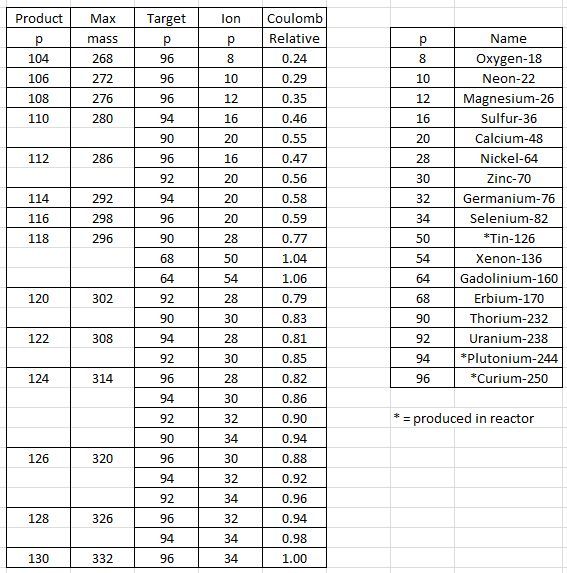
118 Oganesson (symbol Og) was first synthesized in 2002. Two more atoms found in 2005, produced via collisions of californium-249 atoms and calcium-48 ions. Only one isotope known. Isotope 294 decays in 0.89 milliseconds. The -on ending is because it’s in the same group as neon, argon, krypton, xenon, radon.
117 Tennessine (symbol Ts). Fifteen tennessine atoms have been observed: six when it was first synthesized in 2010, seven in 2012, and two in 2014. Both isotopes, 293 and 294, produced via collisions of berkelium-249 atoms and calcium-48 ions. The -ine ending is because it’s in the same group as fluorine, chlorine, bromine, iodine, astatine. Half lives are approximately 22 and 51 milliseconds.
116 Livermorium (symbol Lv). Four isotopes are known, with mass numbers between 290 and 293. Livermorium-293 was first synthesized in 2000, from curium-248 atoms and calcium-48 ions. Between 2004 and 2006 the other three isotopes were found, the lighter two using curium-245 atoms. Half lives, from lightest to heaviest isotopes, are approximately 15, 6, 18 and 53 milliseconds.
115 Moscovium (symbol Mc). Four isotopes are known, with mass numbers between 287 and 290. Half lives, from lightest to heaviest isotopes, are 16, 220, 87 and 32 milliseconds. Isotopes 287 and 288 were first found in 2003, from bombarding americium-243 with calcium-48. The two heavier isotopes of moscovium, 289 Mc and 290 Mc, were discovered in 2009–2010 as daughters of the tennessine isotopes 293 Ts and 294 Ts.