Cymek said:
What I am trying to say is you have a supernova explosion, various elements are created, what size are they though are they all tiny specks or could you get pieces hundreds of tons or more in size and if so wouldn’t they be something you wouldn’t commonly find in planets, asteroids in such size.
Heavy elements are formed by the collapsing and exploding star and then spewed out all over the place, in the form of a dusty cloud. Particles like iron will condense but they soon undergo chemical reactions in the interstellar medium (turning into oxides, sulphides etc).
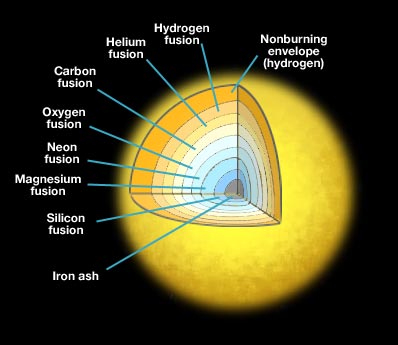
>The nucleosynthesis, or fusion of lighter elements into heavier ones, occurs during explosive oxygen burning and silicon burning processes. Those fusion reactions create the elements silicon, sulfur, chlorine, argon, sodium, potassium, calcium, scandium, titanium and iron peak elements: vanadium, chromium, manganese, iron, cobalt, and nickel. These are called “primary elements”, in that they can be fused from pure hydrogen and helium in massive stars. As a result of their ejection from supernovae, their abundances increase within the interstellar medium.
….All nuclear fusion reactions that produce heavier elements cause the star to lose energy and are said to be endothermic reactions. The pressure that supports the star’s outer layers drops sharply. As the outer envelope is no longer sufficiently supported by the radiation pressure, the star’s gravity pulls its outer layers rapidly inward. As the star collapses, these outer layers collide with the incompressible stellar core, producing a shockwave that expands outward through the unfused material of the outer shell. The pressures and densities in the shockwave are sufficient to induce fusion in that material, and the energy released leads to the star’s explosion, dispersing material from the star into interstellar space.
https://en.wikipedia.org/wiki/Supernova_nucleosynthesis