Tau.Neutrino said:
Here’s The Mind-Boggling Reason The Periodic Table Doesn’t Seem to Have an End
As researchers around the globe race to create the next element on the periodic table – an atom that contains 119 protons – one scientist from Michigan State University is looking into where the table might end.
more…
https://sci-hub.tw/https://www.nature.com/articles/s41567-018-0163-3
The use of ‘hot-fusion’ reactions with neutron-rich 48Ca beams and actinide targets has resulted in measurements of over fifty isotopes of new elements with Z= 114-118 in between 1998 and 2008.
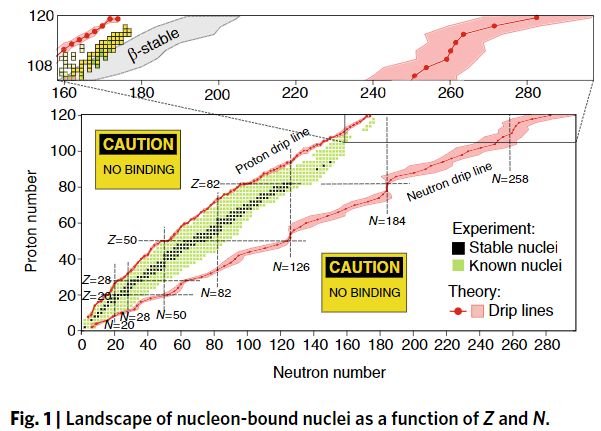
The un-accessed region marked “beta-stable” is what used to be called the second island of stability. This graph is new in that it continues even past N=258.
I see that the gap between the “proton drip line” and “neutron dip line” continues to grow as nuclei get heavier.
“Coming back to Coulomb frustration, one cannot exclude a possibility that there exist isolated islands of nuclear stability, associated with very exotic topologies of nuclear density, stabilized by shell effects. However, it is difficult to say at present whether such exotic topologies can occur as metastable states and what is their stability to various decay modes, especially fission.”
“The pattern of nucleonic shells undergoes significant changes in the superheavy region. The main factors driving these changes are Coulomb frustration effects and the large density of single-particle levels, which grows faster than expected from the A^1/3 scaling. The latter implies that differences in theoretical models can impact the order of nucleonic shells significantly when extrapolating mass and atomic number. From this point of view, it is entirely possible that the nucleus 208Pb is the last ‘proper’ doubly-magic nucleus”.
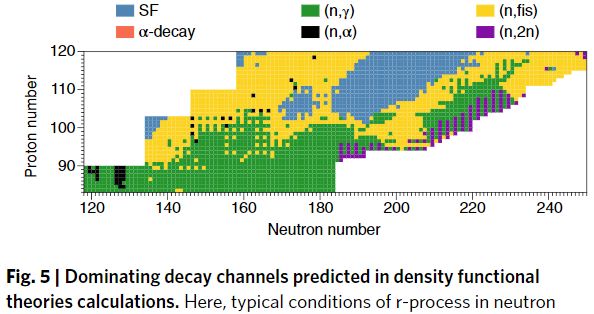
“Unlike in the case of α-decay, there is no theoretical consensus about spontaneous fission lifetimes of superheavy nuclei. This is because predictions are very sensitive to both input (forces, functionals, treatment of collective inertia) and theoretical framework used. Consequently, theoretical lifetime estimates often differ by many orders of magnitude.”
Um, what is “density functional theory” in this context?
References 19 and 21 seem important.
19. Tolokonnikov, S. V., Borzov, I. N., Kortelainen, M., Lutostansky, Y. S. & Saperstein, E. E. Alpha-decay energies of superheavy nuclei for the Fayans functional. Eur. Phys. J. A53, 33 (2017).
21. Giuliani, S. A., Martnez-Pinedo, G. & Robledo, L. M. Fission properties of superheavy nuclei for r-process calculations. Phys. Rev. C.97, 034323 (2018).
Now back to the circling electrons and chemical properties.
“Since atomic relativistic effects scale approximately with Z^2, chemical properties of superheavy atoms cannot be properly described by non-relativistic quantum mechanics. Quantum electrodynamic corrections become substantial. The experimental chemical tests are extremely difficult because one-atom-at-a-time chemistry requires half-lives of the order of 1–2 s and production rates of at least a few atoms per day. There has been major progress in the chemical characterization of transactinides, with the element Fl (Z=114) marking the limit of chemistry today.”
