Tau.Neutrino said:
BREAKING: Complex Organic Molecules Discovered on Enceladus For The First Time
The plumes of salty water shooting out of Saturn’s ocean moon Enceladus have just ponied up one of the most significant ingredients for habitability: large organic molecules rich in carbon.
more…
Given that Cassini is dead, they kept that quiet.
https://www.nature.com/articles/s41586-018-0246-4
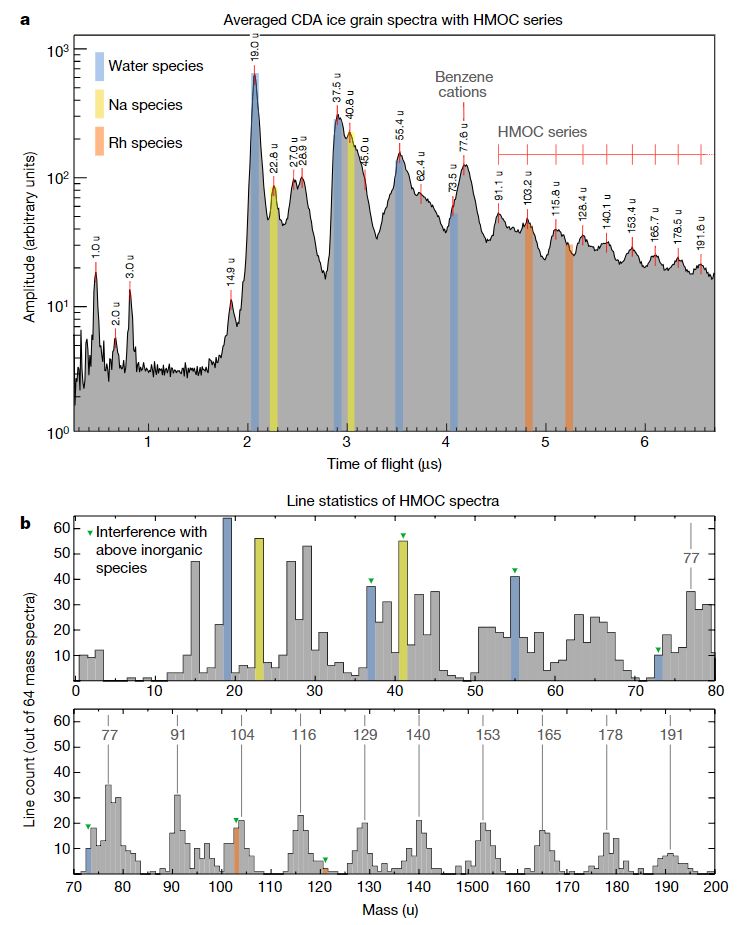
“Identified organic mass lines of individual HMOC spectra.” OK, so they measured mass spectra in both the Enceladus plume and in Saturn’s E ring, not chemical elements individually.
I count the peak at 191 mass units as 15 carbon atoms.
The ratio of carbon to other elements (eg. 1 carbon to 0.73 hydrogen atoms) suggests it has to be either high in double bonds or in rings.
!5 carbon atoms, 191 mass units is much bigger than anything else found in space
https://en.wikipedia.org/wiki/List_of_interstellar_and_circumstellar_molecules#Ten_or_more_atoms_(17)
other than fullerene.